Subtle inflammation: a possible mechanism of future cardiovascular risk in obese children
Article information
Abstract
Purpose
The risk of cardiovascular disease (CVD) has been shown to be associated with systemic inflammation in obese adults with metabolic syndrome (MetS). The aims of this study were to evaluate the prevalence of MetS and its relation to inflammatory markers in obese Thai children.
Methods
A cross-sectional study was conducted. Children with history of endogenous obesity, chronic diseases, drug ingestion, and any acute illness within 2 weeks prior to enrollment were excluded. Their fasting blood glucose (FBG) levels, oral glucose tolerance tests, insulin, lipid profiles, and selected inflammatory markers, including interleukin-6, tumor necrosis factor-alpha, and high-sensitivity C-reactive protein (hs-CRP) levels, were tested.
Results
In this study, 58 obese Thai children (female, 20; male, 38) with a mean body mass index z score of 5.1±2.2 were enrolled. The prevalence of MetS and prediabetes was 31% and 17.2%, respectively. None of the children had diabetes. FBG levels, 2-hour glucose levels, and lipid profiles were not statistically different between those with and without MetS. However, obese children with MetS had higher insulin levels and homeostasis model assessment of insulin resistance values. Elevated hs-CRP levels were found in 69% of the cases, although it was not statistically different between the 2 groups.
Conclusion
We described a substantial prevalence of MetS in Thai obese children. Regardless of MetS status, two-thirds of the obese children had elevated hs-CRP level, indicating subtle ongoing inflammatory process. This chronic inflammation feasibly predisposes them to CVD in the future, even in children without MetS.
Introduction
The prevalence of childhood obesity has drastically increased and become an important global health issue1). If left unconcerned, these children will develop metabolic syndrome (MetS), consisting of central obesity, insulin resistance, dyslipidemia, and hypertension2). This condition has been well recognized and is associated with the low-grade chronic inflammatory process (metainflammation) which is orchestrated by metabolic cells in response to excess nutrients and energy3). Various proinflammatory cytokines and chemokines, such as interleukin-6 (IL-6), tumor necrosis factor (TNF), and monocyte chemo-attractant protein-1, are released in excess fat tissue. They are secreted locally and systemically by visceral adipocytes and induce migration of macrophages into the adipose tissue, which perpetually lead to cytokine release. Not only producing local effects, metainflammation also results in systemic inflammation which plays role in the development of insulin resistance. These inflammatory molecules, including C-reactive protein (CRP), also cause macrophage differentiation, low-density lipoprotein cholesterol (LDL-C) oxidation, and lipid-laden foam formation in the intima of arteries leading to atherosclerotic plaque formation4). This risk is particularly higher when obesity continues from childhood into adulthood, and is found to be markedly increased in the obese patients with MetS5).
Although CRP, regulated by various inflammatory cytokines, principally IL-6 and TNF-α, has been routinely used as an inflammatory marker to determine systemic inflammation, the high-sensitive CRP (hs-CRP) is more sensitive for subtle inflammation467). There are relationships between the elevation of hs-CRP and body mass index (BMI) in obese children and adolescents8). A previous study reported that hs-CRP (>3 mg/L) was associated with an increased 10-year risk of coronary heart disease, regardless of the presence or absence of cardiovascular risk factors9). Subjects being in the highest quartile of hs-CRP would experience the adjusted relative risk of 2.3 to 4.8 times for cardiovascular diseases (CVDs) compared with those in the lowest quartile4). Subsequent studies showed that hs-CRP levels were higher in adolescents with MetS than in those without MetS1011). Moreover, elevation of hsCRP was reported to be associated with carotid artery media thickness in adolescents indicating early atherosclerotic plaque formation1112). We hypothesized that obesity is associated with systemic inflammation and this association is stronger in those having MetS. This study aims to determine the prevalence of MetS and the association between inflammatory markers and MetS in obese Thai children.
Materials and methods
This was a cross-sectional study, enrolling children and adolescents who were 6–8 years old with BMI greater than the 95th percentile by age and sex according to an international cutoff for BMI. They were diagnosed as exogenous obesity. Children with history of endogenous obesity (Prader-Willi syndrome, Cushing syndrome), chronic diseases (primary hyperlipidemia, asthma, diabetes mellitus, hypertension), drug ingestion and acute illnesses within 2 weeks were excluded.
Fasting blood glucose, insulin, lipid profiles, hs-CRP, IL-6, and TNF-α were obtained. Oral glucose tolerance tests (OGTTs) was carried out using glucose 1.75 g/kg, max 75 g, in which the blood glucose and insulin were repeatedly taken at 2 hours. The hs-CRP was measured by the electrochemiluminescent method, whereas IL-6 and TNF-α were measured by the enzyme-linked immunosorbent assay. The index of insulin resistance using the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated.
Participants were subsequently categorized into 2 groups based on MetS status, using the modification of the definition of the MetS proposed by the International Diabetic Federation (IDF)13). Patients with MetS had to meet obesity diagnostic criteria and possessed at least two more of the following criteria, including triglyceride≥150 mg/dL, high-density lipoprotein cholesterol (HDL-C)<40 mg/dL, systolic blood pressure≥130 mmHg or diastolic blood pressure≥85 mmHg, and fasting blood glucose≥100 mg/dL. Prediabetic patients were considered when they had impaired fasting glucose (100–125 mg/dL) or impaired glucose tolerance (IGT) (140–199 mg/dL) at 2-hour during OGTT, whereas diabetes mellitus will be diagnosed if either the fasting blood glucose≥126 mg/dL or 2-hour blood glucose≥200 mg/dL during an OGTT14). A diagnosis of hyperlipidemia was defined as follows: triglyceride>150, total cholesterol>200, and LDL-C>130 mg/dL. HDL-C was considered low if its level was below 40 mg/dL13). Finally, insulin resistance was diagnosed if the fasting insulin≥15 µU/mL or 2-hour insulin during OGTT≥75 µU/mL15) or HOMA-IR>3.16 was observed16). There was no cutoff value or normal range available for IL-6 and TNF-α reported. However, hs-CRP>3 mg/L was considered to be clinically elevated9).
This project was approved by the Ethics Committee of Chiang Mai University hospital (approval number: PED-11-08-22A-13) and fully supported by the Faculty of Medicine Endowment Fund of the Faculty of Medicine of Chiang Mai University, Chiang Mai, Thailand. Written informed consents were obtained from the parents or guardians for all participants.
For comparison between groups, the Mann-Whitney U test was used for continuous variables, whereas chi-square and Fisher exact test were applied for categorical variables. Statistical analysis was performed using SPSS ver. 17 (SPSS Inc., Chicago, IL, USA). A P value of <0.05 was considered statistically significant.
Results
1. Patient characteristics
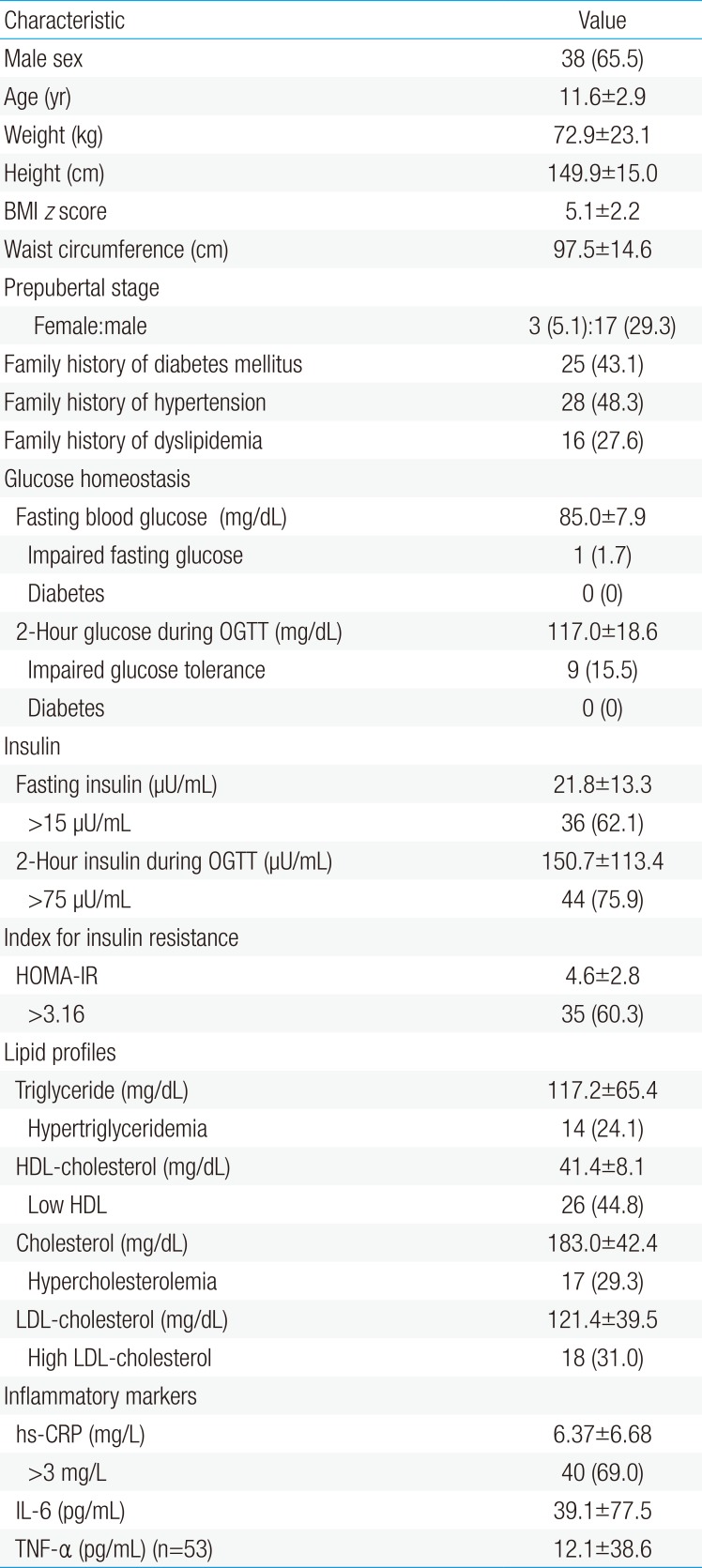
Fifty-eight obese children and adolescents, 38 males (65.5%) and 20 females (34.5%), with a mean age and BMI z score of 11.6±2.9 years old and 5.1±2.2, respectively, were enrolled. Twenty participants (34.4%) were in the prepubertal stage. Table 1 summarizes the characteristics of the study participants.
2. Glucose, insulin resistance, and lipid profiles
The mean of the fasting blood glucose and 2-hour glucose during OGTT were 85.0±7.9 and 117.0±18.6 mg/dL, respectively. Regarding diabetic status, 17.2% of participants were defined as prediabetes (impaired fasting blood glucose, 1.7%; IGT, 15.5%). No subject met the criteria diagnosis of diabetes. The mean of the fasting insulin was 21.8±13.3 µU/mL and the mean of the 2-hour insulin during OGTT was 150.7±113.4 µU/mL. An elevated fasting insulin, 2-hour insulin during OGTT, and HOMA-IR were identified in 62.1%, 75.9%, and 60.3%, respectively. Dyslipidemia, including hypertriglyceridemia, hypercholesterolemia, high LDL-C, and low HDL-C, were identified in 24% to 45% of the cases (Table 1).
3. Metabolic syndrome and inflammatory markers
The prevalence of MetS was 31%. The average value of hs-CRP was 6.37±6.68 mg/L, in which 69% of the participants showed elevated hs-CRP level. The mean of IL-6 and TNF-α were 39.1±77.5 pg/mL and 12.1±38.6 pg/mL, respectively.
4. Clinical and biochemical data in children with and without MetS
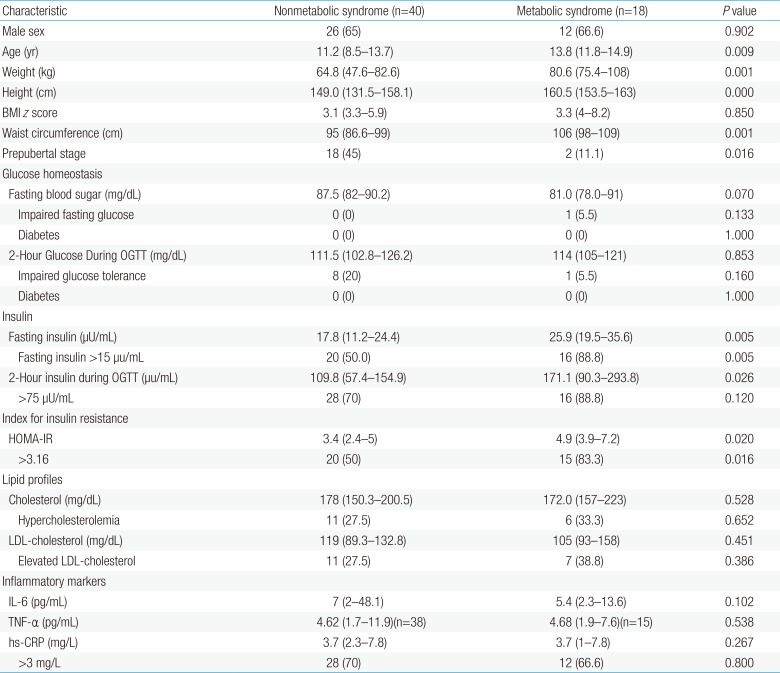
Obese children with the MetS were older (13.8 years vs. 11.2 years, P=0.009) than those without MetS, but comparable BMI z score (P=0.850). The fasting insulin (median [interquartile range], 25.9 [19.5–35.6] µU/mL vs. 17.8 [11.2–24.4] µU/mL, P=0.005), 2-hour insulin during an OGTT (171.1 [90.3–293.8] µU/mL vs. 109.8 [57.4–154.9] µU/mL, P=0.026), and HOMA-IR (4.9 [3.9–7.2] vs. 3.4 [2.4–5], P=0.020) were also significantly higher in obese children with MetS. However, the fasting blood glucose, 2-hour glucose during an OGTT, and lipid profiles were not statistically different between the groups. Regarding inflammatory markers, hs-CRP, IL-6, and TNF-α were also not statistically different between 2 groups. However, the overall percentage of elevated hs-CRP were exceptionally high, even in obese children without MetS (Table 2)
Discussion
This study demonstrated a high prevalence of metabolic syndrome and elevated hs-CRP among obese Thai children. As the elevation of hs-CRP level is suggestive of subtle inflammatory process, our finding may help increase awareness of CVD risk even in obese children without MetS.
Defining MetS in children is rather difficult, particularly in children due to pubertal effects and the low prevalence of CVD2). As a result, there have currently been no definite consensus guidelines providing specific diagnostic criteria for pediatric MetS17). However, there have been several inconsistent criteria, proposed by several authorities and organizations including the IDF, National Cholesterol Education Program Adult Treatment Panel III1718). Yet, the use of waist circumference was not included in the pediatric criteria at the present time due to insufficient information and lack of specific guidance for clinical application, according to an expert committee of the American Medical Association and the Centers for Disease Control and Prevention Task Force on Assessment, Prevention, and Treatment of Childhood Obesity19). Additionally, reference values of waist circumference for Thai children has not been available. Therefore, we decided to exclude this parameter and employ the assessment on the MetS status with the modified MetS criteria proposed by IDF, in which the prevalence of MetS in our study was 31%. It was comparable to other reports and ranged from 16.9%–38.5%202122).
Although there is a concept of the healthy obesity which has been believed not associated with the CVD risk, the diagnostic criteria of MetS have not included inflammatory markers as well as features of nonalcoholic fatty liver disease2324). The presence of subclinical inflammations could play a key role in distinguishing metabolically healthy from metabolically nonhealthy individuals24). Although there were no statistically significant differences between participants with and without MetS, we demonstrated the incredibly high prevalence of cases with elevated hs-CRP level, indicating the presence of low grade inflammatory process which possibly predispose to CVD in both obese children and adolescents. This finding challenges the concept of the healthy obesity and could feasibly lead to the inclusion of inflammatory markers in the criteria diagnosis of MetS.
Our results were in alignment with previous studies, demonstrating that obese children had elevated inflammatory markers, for instance hs-CRP82526272829303132), TNF-α26272933), and IL-626,28,34). Compared to the study done by El-shorbagy and Ghoname25) demonstrating a prevalence of hs-CRP>3 mg/L of 35%, our study showed as much as twice higher prevalence. Unfortunately, we could not identify MetS as a potential risk for increasing the selected inflammatory cytokines even though there was a report to that effect35). This may reflect racial difference and severity obesity influence on metainflammation among different populations.
There were several limitations in our study. Firstly, our study did not have a matched control nonobese group due to the ethical reason. Secondly, our study had a small sample size with heterogeneous age and pubertal status, which could affect the insulin resistance and MetS status. Lastly, this study was a cross-sectional study while MetS and atherosclerosis are chronic processes. Therefore, the long-term follow up study of these children is needed.
In summary, we described a high prevalence of elevated inflammatory markers in obese children. Nonetheless, there was no statistical difference of hs-CRP level in obese children with or without MetS. This piece of information affirms existing knowledge regarding the presence of subtle chronic inflammatory process in obese children. If left unrecognized, it could be one of predisposing factors of CVD in their future life, so early weight intervention should be emphasized. Further studies are still needed to firmly endorse these clinical implications and whether an increase in inflammatory markers should be integrated as a parameter in the criteria diagnosis of MetS.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.