Effects of clarithromycin treatment in scrub typhus in children: comparison with chloramphenicol and azithromycin
Article information
Abstract
Purpose
Chloramphenicol and tetracycline are not recommended for treating scrub typhus in pediatric patients because of potential side effects, such as aplastic anemia or tooth discoloration. While clarithromycin has recently been used in adults, few reports have been published on its effects in pediatric patients. We report the clinical profiles of pediatric scrub typhus and the effects of clarithromycin on scrub typhus in children.
Methods
We retrospectively analyzed medical records of 56 children with scrub typhus who were admitted between 2004 and 2013 to Chonbuk National University Hospital, Jeonju, Korea. Cases were divided into 3 groups based on thetreatment drug (chloramphenicol, azithromycin, and clarithromycin). We compared their clinical manifestations and laboratory findings.
Results
All patients exhibited fever and rash. Other common clinical manifestations were eschars (66%), lymphadenopathy (48%), upper respiratory symptoms (42%), abdominal pain (32%), and hepatosplenomegaly (14%). Elevated levels of C-reactive protein, erythrocyte sedimentation rates, aspartate transaminase, and alanine transaminase were detected in 95%, 96%, 84%, and 77% of patients, respectively. Additionally, decreased platelet and white blood cell levels were observed in 43% and 36% of patients, respectively. There were no statistical differences between the treatment groups in mean age (P=0.114) or sex (P=0.507). However, time to defervescence after the treatments differed significantly, being the shortest in the clarithromycin group (P=0.019). All patients recovered without complications related to the disease or drugs.
Conclusion
Clarithromycin was as effective as chloramphenicol and azithromycin in pediatric scrub typhus patients and may be used as a first-line treatment drug.
Introduction
Scrub typhus, also known as tsutsugamushi disease, is an acute febrile disease caused by Orientia tsutsugamushi, a gram-negative obligate intracellular bacterium in the family Rickettsiaceae. A rapid increase in the incidence of patients with tsutsugamushi disease was observed from 2001 to 2013, with the majority of cases reported in South Korea. Incidence usually increases in children each year in the autumn as a febrile disease1). Conventional treatment includes broad-spectrum antibiotics such as doxycycline, chloramphenicol, and tetracycline, all of which are effective for the treatment of scrub typhus. In pediatric patients, however, chloramphenicol and tetracycline are not recommended because of their potential side effects, such as aplastic anemia or teeth discoloration. New macrolide antibiotics have been found to be effective in animal studies and in clinical scrub typhus infection in children and pregnant women23). Clarithromycin has recently been used for the alternative treatment of scrub typhus in adults, although there are few reports on its effects in pediatric patients4). We evaluated the effects of clarithromycin on scrub typhus treatment compared with chloramphenicol and azithromycin in pediatric patients.
Materials and methods
We retrospectively analyzed the medical records of 56 children with scrub typhus who were admitted to Chonbuk National University Hospital, Jeonju, Korea, between 2004 and 2013. They were divided into a chloramphenicol-treated group (CM group), an azithromycin-treated group (AZ group), and a clarithromycin treated group (CL group). We compared the clinical manifestations and laboratory findings among the 3 groups. The diagnosis of scrub typhus was made by characteristic clinical manifestations with eschar and/or a positive serological test. Clinical manifestations were fever, cough, skin rash, gastrointestinal symptoms, myalgia, lymphadenopathy, abdominal pain, hepatomegaly, splenomegaly, and hematuria. Fever was defined as a body temperature of 38.0℃ or higher.
Laboratory data included platelet counts, white blood cell counts, hemoglobin levels, and serum levels of C-reactive protein (CRP), aspartate transaminase (AST), and alanine transaminase (ALT). The serologic diagnosis of scrub typhus was made when an indirect immunofluorescent antibody assay IgM titer against O. tsutsugamushi was ≥1:80.
Patients were treated in one of the following ways: CM group: A 50 mg/kg/day CM divided dose every 6 hours for 7 days (oral or intravenous); AZ group: A 10 mg/kg dose of AZ on day 1, followed by 5 mg/kg daily for 4 more days (oral); or CL group: A 15–30 mg/kg/day CL divided dose every 12 hours for 5 days (oral). To evaluate the therapeutic effect, we compared the 3 groups according to hospitalization period, time to defervescence after treatment, and complications.
The data were analyzed using descriptive statistics and one-way analysis of variance with GraphPad Prism 5.0 software (GraphPad, La Jolla, CA, USA). A P value <0.05 was considered statistically significant.
Results
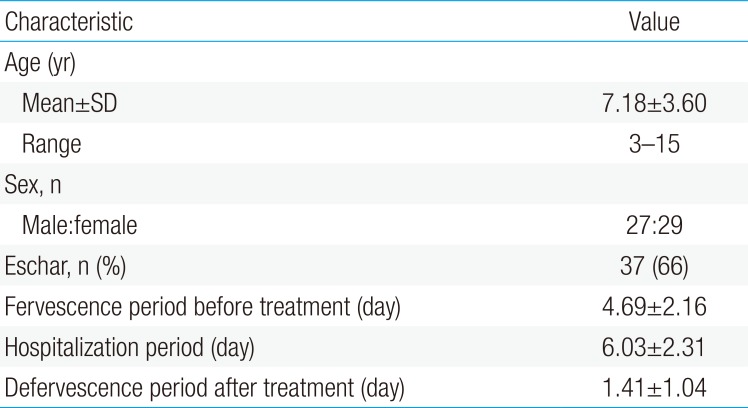
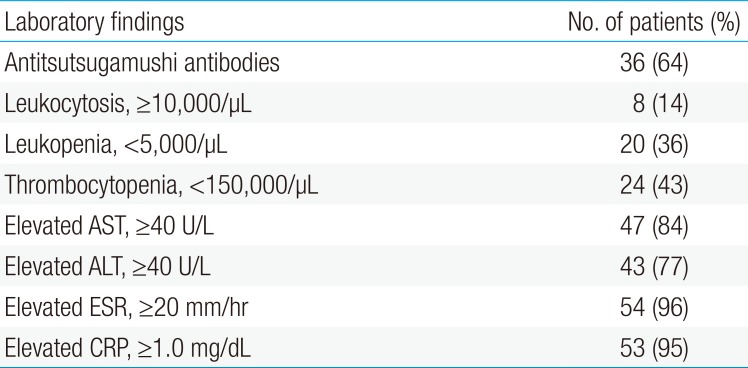
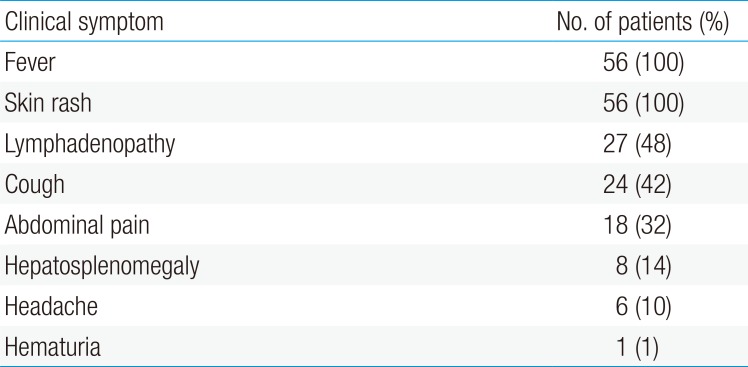
Fifty-six scrub typhus cases diagnosed from 2004 to 2013 were selected. Mean age was 7.18 years (range, 3 to 13 years); 27 children were male and 29 were female. Thirty-seven cases (66%) were diagnosed by eschar on the body (Table 1). Thirty-six cases (64%) were serologically confirmed with an antitsutsugamushi antibody test (Table 2). All patients had a fever (>38℃) and erythematous skin rash. Other common clinical manifestations were lymphadenopathy (48%), cough (42%), abdominal pain (32%), and hepatosplenomegaly (14%) (Table 3).
Several laboratory values were consistently elevated among cases, including CRP, erythrocyte sedimentation rate, AST, and ALT. These were detected in 95%, 96%, 84%, and 77% of the patients, respectively. In addition, decreased counts of platelets and white blood cells were seen in 43% and 36% of the patients, respectively (Table 2). There were no statistical differences between the groups in mean age (P=0.114) or sex (P=0.507). Time to defevervescence after the treatment was 1.26±0.30 days in the CM group, 1.80±0.15 days in the AZ group, and 0.83±0.20 days in the CL group. The differences between the groups were statistically significant (P=0.019). In addition, the mean duration of hospitalization in the CL group was shorter than that in the CM and AZ groups (P=0.009) (Table 4).
All patients recovered without complications related to the disease or the drugs. No mortalities occurred in the cohort. Moreover, no relapses were reported in any of the treatment groups during a 1-month follow-up period.
Discussion
Scrub typhus, caused by O. tsutsugamushi is a febrile and infectious illness endemic to Southeast Asia and Australia5). In Korea, scrub typhus was first reported in 6 United Nations (UN) soldiers stationed in the country during the Korean War6). Since then, the overall prevalence rate of the disease has been on the rise in Korea; even in pediatric patients, its occurrence as a febrile illness increases each year in autumn1). The infection route in pediatric patients is believed to be direct exposure to trombiculid mites during outdoor activities, and recent increases in outdoor activities may have led to a higher risk of exposure. It appears that global warming may be influencing host reproduction and increasing the rate of proliferation and the period of transmission. This would account for the growing prevalence of the disease7). Moreover, the incidence rate of scrub typhus in Korea begins to increase in September, reaches its peak in November, and decreases in December; this trend coincides with the period of increasing trombiculid mites, the vector of the disease. The cases in this report occurred in autumn, mainly in November.
When patients show distinctive symptoms such as rash and eschar with a history of outdoor activities, they can be clinically diagnosed with the disease. However, in many cases, it is difficult to diagnose scrub typhus using only clinical symptoms, as rash and eschar are not always present in scrub typhus patients. In such cases, serological diagnosis, polymerase chain reaction assays, and bacterial culturing are alternatives methods of making a diagnosis.
Serological diagnosis is the diagnostic standard for scrub typhus. Although the indirect immunofluorescence test has a high sensitivity and specificity, this type of serum test offers a relatively low clinical utility, as it is generally used to confirm the disease after treatment has started. Scrub typhus antibodies are usually detectable 2–3 weeks after the onset of the disease. Thus, the diagnosis of scrub typhus is presumed based on epidemic season or clinical symptoms, and treatments begin before test results are available8). An eschar is a 5–10 mm black scab that forms at the site of the trombiculid bite. The eschar is an important sign for the diagnosis of scrub typhus; as it is a symptom specific to scrub typhus, clinical diagnosis can be made even prior to serological diagnosis9). Although the eschar does not cause any pain or itching, some patients may feel tenderness in lymph nodes because of lymphatic drainage. Therefore, when scrub typhus is suspected, a meticulous full-body inspection of patients' lymph nodes and skin is required
Scrub typhus is transmitted to humans by the bite of infected trombiculid mites known as “chiggers” that live on mice and rats. Scrub typhus causes multisystem vasculitis that involves the brain, skin, liver, lung, and kidney, contributing to a variety of clinical symptoms10).
In the early stage of the disease, many patients experience a high fever, myalgia, and respiratory symptoms, and may misperceive their condition as an upper respiratory infection. In children, scrub typhus is thus difficult to recognize early, and is often diagnosed late. When scrub typhus is identified early, its symptoms are not severe and it can be treated easily with antibiotics. However, when diagnosed late, it causes systemic vasculitis from complications, which in turn can lead to pneumonia, acute renal failure, encephalomeningitis, upper gastrointestinal bleeding, and multiorgan failure. In severe cases, the complications can even result in death9).
While doxycycline and chloramphenicol are well known to beselective for scrub typhus, doxycycline may stain teeth in children, and its use is generally limited in children under the age of eight311). Chloramphenicol poses a risk of aplastic anemia and bone marrow suppression, requiring caution in pediatric use12). Quinolone has been reported as effective in treating scrub typhus in some cases13). However, a few studies have suggested that severe scrub typhus cases should not be treated with quinolone due to resistance and prolonged defervescence1415). Furthermore, quinolone use in children is limited due to adverse musculoskeletal effects.
Suitable medications are therefore needed for treating scrub typhus in pediatric patients. Recently, new macrolide antibiotics have been reported to be effective in treating various rickettsial infections in in vitro experiments, animal testing, and clinical trials46111216). Moreover, the macrolide antibiotics clarithromycin and azithromycin have been verified as effective against O. tsutsugamushi strains resistant to doxycycline12). Recent studies have reported using azithromycin and roxithromycin to treat pediatric patients with scrub typhus58).
The present study aimed to investigate the efficacy of clarithromycin, a new macrolide antibiotic, which is relatively safe to use on children and shows good compliance. We found that clarithromycin was as effective as chloramphenicol or azithromycin in pediatric scrub typhus patients, and may be used as a first-line treatment drug.
This study has a few limitations. It involved only a small number of scrub typhus infection cases, and all were treated as inpatients. However, it is the first study to compare the efficacy of chloramphenicol, representing conventional treatment, and azithromycin and clarithromycin, representing newer alternative treatments.
In conclusion, a determination of treatment options for pediatric patients should consider not only the effectiveness of medications but also their side effects. In the treatment of scrub typhus infection, we can anticipate effective treatment outcomes with macrolide antibiotics, which have relatively fewer side effects than doxycycline and chloramphenicol.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.