Etiology and clinical characteristics of fever of unknown origin in children: a 15-year experience in a single center
Article information
Abstract
Purpose
Fever is one of the most common symptoms in children. In previous studies, infectious disease was the most common cause of pediatric fever of unknown origin (FUO). The aim of this study is to investigate the etiology, clinical characteristics and prognosis of pediatric FUO in 21 century with more diagnostics available and to analyze the factors for certain disease categories.
Methods
Among the children under 18 years old who were hospitalized at Samsung Medical Center from January 2000 to December 2014, the patients who met the criteria including fever of ≥38.0℃ for longer than ≥14 days and failure to reach a diagnosis after one week of investigations were included.
Results
Total 100 patients were identified. Confirmed diagnosis was achieved in 57 patients (57%). Among them, infectious diseases (n=19, 19%) were most common, followed by connective tissue diseases (n=15, 15%), necrotizing lymphadenitis (n=8, 8%), and malignancies (n=7, 7%). Children with fever duration over 28 days had a trend for higher frequency of connective tissue diseases (28.3%) except undiagnosed etiology. The symptoms such as arthritis, lymph node enlargement and only fever without other symptoms were significantly related with connective tissue diseases, necrotizing lymphadenitis and undiagnosed respectively (P<0.001). Ninety-two patients have become afebrile at discharge and 1 patient died (1%).
Conclusion
Almost half of our patients were left without diagnosis. Although it has been known that infectious disease was most common cause of pediatric FUO in the past, undiagnosed portion of FUO have now increased due to development of diagnostic techniques for infectious diseases.
Introduction
Fever is the most common symptom in children. Fever of unknown origin (FUO) was first described in 1961 and was defined as fever with a body temperature ≥38.0℃ for at least 3 weeks duration with a failure to reach a diagnosis after 1 week of inpatient investigation or 3 outpatient visits1). Most viral febrile episodes usually resolve within 1 week and parents typically take their children to the hospital when the fever lasts more than 7 days. Therefore, in recent studies, definitions of pediatric FUO have tended to include patients with unexplained fever that persists longer than 1 or 2 weeks123).
The current incidence of pediatric FUO varies among studies. Cho et al.1) reported the incidence of pediatric FUO is between 0.5% to 3% and Antoon et al.4) reported its incidence remain unclear. It is well-known that the most common causes of pediatric FUO are infectious diseases followed by connective tissue diseases (CTD) and malignancies15). However, the causes of FUO have changed over the years and have been influenced by diagnostic techniques. Due to the development of improved diagnostic techniques, the proportion of FUO caused by infectious diseases has tended to decrease and the proportion of CTD, malignancies, and other diseases has tended to increase34).
There has been no published research on the etiologies and clinical characteristics of pediatric FUO in Korea since 20063). The aims of this study are to investigate the etiology, clinical characteristics, and prognosis of pediatric FUO and to analyze the factors that can predict certain disease categories.
Materials and methods
From January 2000 to December 2014, children younger than 18 years of age who were evaluated for prolonged fever at Samsung Medical Center, Seoul, South Korea, were identified. Among them, the patients who met the inclusion criteria of fever ≥38.0℃ for ≥14 days and no diagnosis after 1 week of investigations were included in this study. Only inpatient patients were included and the patients who had underlying diseases such as immunodeficiency and known malignancies were excluded. Axillary temperature was used in our hospital.
Retrospective chart review was performed to collect the patients' characteristics including age, sex, duration of fever, past history, symptoms, physical examination findings, laboratory results, treatment (if given), hospital course, hospitalization period, and prognosis. For counting the duration of fever, we defined defervescence as being afebrile more than 2 days and maintain afebrile condition until discharge. Outside hospital data were also included for the duration of fever and hospitalization period. Laboratory data included complete blood cell count, chemistry profiles with liver function tests and kidney function tests, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), urinalysis, and urine and blood cultures. Further examinations such as autoimmune markers, immunologic work-up, viral and bacterial studies by serology tests and/or polymerase chain reaction (PCR), tuberculin skin test (TST) or interferon gamma releasing assay (IGRA), and cerebrospinal fluid (CSF) exam were performed based on the clinical context. Imaging studies including chest radiography, ultrasonography, computed tomography (CT), echocardiography, bone scan, magnetic resonance imaging (MRI), and positron emission tomography (PET) were performed according to the patients' clinical presentations. Invasive procedures such as bone marrow examination and other tissue biopsies including those from lymph node biopsy, bronchoscopy, esophagogastroduodenoscopy, and colonoscopy were performed when indicated.
The causes of FUO were categorized into 6 groups: infectious diseases, CTD, malignancies, necrotizing lymphadenitis, miscellaneous, and undiagnosed. Data were analyzed by IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA) and statistical analysis was performed using the Fisher exact test for categorical variables. To analyze the continuous variables (CRP, WBC, and ESR), Kruskal-Wallis test with Bonferroni correction was used. A value of P<0.05 was considered statistically significant.
Results
A total of 392 patients were evaluated for prolonged fever and 292 patients were excluded for the following reasons: duration of fever less than 14 days, total hospitalization less than 7 days, and diagnosis reached before 1 week of investigation. As a result, 100 patients were included in this study.
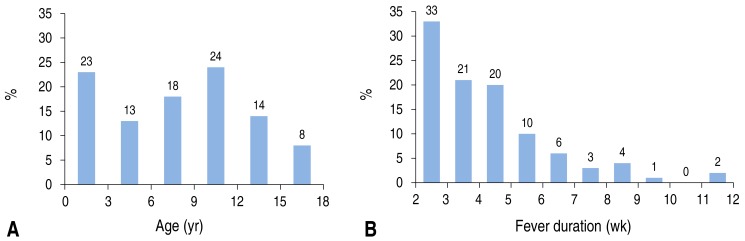
Table 1 shows the characteristics of the patients included in this study. There were 65 boys (65.0%) and the median age was 8.5 years (range, 0.1–17.6 years). Detailed data on the age distributions and fever durations are shown in Fig. 1. Eight patients (8%) were less than 1 year old; among these patients, the diagnosis of 3 patients were confirmed to be atypical Kawasaki disease, hemophagocytic lymphohistiocytosis (HLH), and hereditary sensory-autonomic neuropathy (HSAN), and the remaining 5 patients were undiagnosed. These 5 undiagnosed patients less than 1 year old recovered from fever without any sequelae. In all patients, the median fever duration was 27.5 days (range, 14–80 days). The patient with the longest fever became afebrile without a definite diagnosis after 80 days of fever. This patient was a 17-year-old girl with a headache who was transferred from an outside hospital where she received extensive FUO evaluation for infection, autoimmune disease, and malignancy, including bone marrow examination. After hospitalization in our hospital, similar investigations were performed again. Her fever subsided during the hospitalization without a definite diagnosis. The median duration of hospitalization was 24 days (range, 7–110 days) and the patient with the longest hospitalization was later diagnosed with lymphoma after 3 hospitalizations. She presented with fever and recurrent upper respiratory symptoms and had a family history of lymphoma in an older brother who died at an age of 5 years. Additional image work-ups showed multifocal bowel wall thickening with mesenteric lymphadenopathy, which suggested a lymphoproliferative disorder. Initial colon biopsy showed benign lymphadenopathy with extensive plasmacytic infiltration. However, repeat colon biopsy 2 months later in the setting of persistent fever revealed lymphoma transformation.
The final diagnoses for FUO are shown in Table 2. Fifty-seven patients (57.0%) had a confirmatory diagnosis and 43 patients (43.0%) remained undiagnosed. Among the patients with a confirmatory diagnosis, infectious diseases were the most common causes of FUO (n=19, 19.0%), followed by CTD (n=15, 15.0%), necrotizing lymphadenitis (n=8, 8.0%), miscellaneous (n=8, 8.0 %), and malignancies (n=7, 7.0%).
Among the patients who were diagnosed with infectious diseases, viral infection was the most common (n=8, 42.1%). Pathogens and clinical diagnoses are shown in Table 3. Half of viral infections were respiratory tract infections and 1 patient had a coinfection of a respiratory tract infection caused by respiratory syncytial virus (RSV) and a urinary tract infection. The most common viral pathogen was RSV (n=4). All of the diagnoses of respiratory virus infections were made by culture before the PCR results were available. Among these, 3 patients were referred from an outside hospital after more than 7 days of hospitalization. Only 1 patient had obvious respiratory symptoms. In 1 patient with meningitis, CSF WBC was 24/µL, protein was 25.4 mg/dL, glucose was 53 mg/dL and serum glucose was 92 mg/dL. Enterovirus was considered to be the responsible pathogen even when enterovirus was only recovered in the stool. There were 3 cases of Mycobacterium tuberculosis (n=3, 15.8%) infection: tuberculous meningitis, endobronchial tuberculosis, and tuberculous cervical lymphadenitis. These three cases of M. tuberculosis infection were diagnosed by positive mycobacterium culture in the CSF, sputum, and lymph node, respectively. In all 3 patients treated for tuberculosis, the TST was negative and there was no definite tuberculosis exposure history. Two patients were diagnosed with infective endocarditis and received intensive care. Methicillin-sensitive staphylococcus aureus was recovered from the blood culture of one patient, while the other patient had a blood culture that was negative, but bacterial DNA identification PCR of heart vegetation confirmed the presence of Haemophilus parainfluenzae. There was 1 patient with culture-negative septic arthritis who received empiric antibiotic treatment before proper cultures were obtained at an outside hospital because the patient was in septic shock and was transferred to our intensive care unit. The patient recovered with broad-spectrum antimicrobial treatment in our hospital.
Fifteen patients (15.0%) were diagnosed with CTD. Among these, 6 patients (40%) were boys and 9 patients (60%) were girls. In this group with CTD, the number of female patients was significantly higher than the number of male patients (P=0.028). Six patients had positive antinuclear antibody (ANA) and all 3 patients who were diagnosed with systemic lupus erythematosus (SLE) had both positive ANA and antidouble stranded DNA (anti-ds DNA).
Eight patients (8.0%) were diagnosed with necrotizing lymphadenitis by lymph node biopsy. Seven patients had cervical lymph node enlargement and 1 patient had axillary lymph node enlargement.
Seven patients (7.0%) were diagnosed with malignancies. Among the 3 patients with lymphoma, 2 cases of lymphoma were diagnosed by colon biopsy with final diagnoses of mature T-cell lymphoma and mucosa-associated lymphoid tissue lymphoma, respectively. One lymphoma patient was diagnosed by inguinal lymph node biopsy. Two patients with neuroblastoma presented only with leg pain and were initially suspected to have juvenile idiopathic arthritis (JIA), but had persistent leg pain and fever. Bone biopsy was performed in 1 patient, with a result suggesting osteomyelitis or bone tumor. Further imaging studies were performed and a paravertebral mass was founded. Neuroblastoma was diagnosed by paravertebral mass biopsy. In the other patient, knee MRI was performed and multifocal osteonecrosis and a bone marrow signal change was observed. Additional evaluation was performed and a suprarenal mass was found on both abdomen ultrasonography and CT. Finally, neuroblastoma was diagnosed by mass biopsy. There were 2 cases of HLH that were diagnosed by bone marrow biopsy.
Among the patients with miscellaneous diagnoses, there was 1 case of HSAN, which is a congenital disorder characterized by anhidrosis, fever, and a loss in the ability to feel pain or sense hot and cold. We suspected this genetic disorder when we observed that the patient did not cry during the blood draw and learned about the patient's history of anhidrosis from the parents when they were asked specifically about sweating. HSAN was confirmed by gene study (NTRK1 gene mutation). There was 1 case of chronic recurrent multifocal osteomyelitis, which is a form of nonbacterial osteomyelitis that primarily occurs in childhood. Its presentation includes periodic bone pain, fever, and the appearance of multiple bone lesions that can occur in any skeletal site. This patient with chronic recurrent multifocal osteomyelitis was an 11-year-old girl who suffered from bilateral knee pain. Her MRI showed diffuse bone marrow signal change in both tibias and suspected osteomyelitis or leukemic infiltration. Further investigations including bone and bone marrow biopsy were performed. Bone biopsy revealed fibrotic change with inflammatory cells and bone marrow biopsy showed no abnormal leukemic cells. Bone and bone marrow cultures for bacteria, fungi, and mycobacteria were negative, and the acid-fast bacilli stain and mycobacterial PCR were negative. TST was negative but the initial IGRA was positive. Therefore, she was suspected of having tuberculous osteomyelitis and was treated with a 4-drug antituberculosis regimen (isoniazid, rifampin, ethambutol, and pyrazinamide). Her symptoms initially responded to treatment, but her fever and bone pain continued to wax and wane throughout her follow-up in outpatient clinic without radiological improvement after 2 years of medication. Mycobacterium was not isolated from any lesions and there was no evidence of drug-resistant tuberculosis. During her follow-ups, additional IGRA test results were negative once and indeterminate twice. Antituberculosis drugs were discontinued after 2 years of treatment. Other possibilities were pursued and follow-up MRI, PET, and bone scans showed persistent bone marrow lesions involving multiple bones. Finally, chronic recurrent multifocal osteomyelitis was suspected and the patient was placed on steroid therapy, which provided symptom relief. After 3 years of steroid therapy, she stopped the medication.
Table 4 shows the relationship between the age of onset, duration of fever, and the etiologies. Except undiagnosed etiology, among the patients who were ≥6 years old, CTD had the highest percentage (20.3%, 13 of 64). Among the patients who were <6 years old, infectious disease had the highest percentage (22.2%, 8 of 36). Except undiagnosed etiology, among the patients who had fever>28 days, CTD had the highest percentage (28.3%, 13 of 46) and among the patients who had fever ≤28 days, infectious disease had the highest percentage (22.2%, 11 of 54). The median value of duration of fever is the highest in CTD group (42 days).
The relationship between the symptoms and etiologies of FUO are shown in Fig. 2. The group with FUO due to infectious diseases had more respiratory symptoms such as cough, sputum, and rhinorrhea than the other groups, but this was not statistically significant. The CTD group had more symptoms of arthralgia than the other groups (46.7% vs. 9.4%, P=0.001). In the undiagnosed group, the proportion of patients who had fever without any other symptoms was higher than in the other groups, which was statistically significant (42.0% vs. 7.0%, P<0.001).
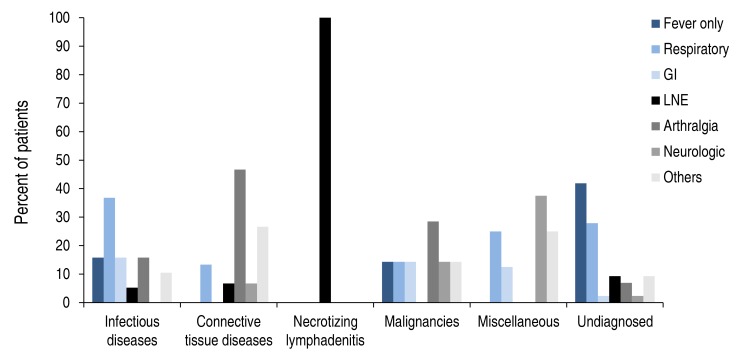
The relationship between symptoms and final diagnosis in fever of unknown origin. Abdominal pain, vomiting or diarrhea refer to gastrointestinal (GI) symptoms; Cough, sputum or rhinorrhea refer to respiratory symptoms; Lymph node enlargement (LNE) includes cervical or axillary LNE; Neurologic symptoms contained headache, seizure, gait disturbance or anhidrosis; Others contained skin rash, oral ulcer, oral vesicle or conjunctival injection.
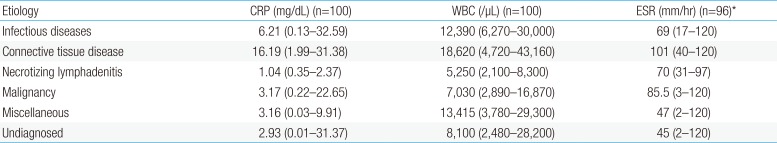
Table 5 shows the relationships between inflammatory markers (CRP, WBC, ESR) and the etiologies of FUO. The maximum values of CRP, WBC, and ESR of individual patients were retrieved and compared among the 6 groups. The CRP level of the CTD group was significantly higher than that in the necrotizing lymphadenitis (P=0.001) and undiagnosed group (P<0.001). The WBC count of the CTD group was significantly higher than that in patients who were undiagnosed (P=0.003) or who had necrotizing lymphadenitis (P<0.001). The ESR levels in the CTD group were significantly higher than those in the undiagnosed group (P=0.001).
Many invasive diagnostic tests were used. Among these invasive procedures, bone marrow biopsy was performed in 40 patients (40%) and biopsies of other sites including the lymph node, joint, bone, bronchus, heart, and colon were performed in 33 patients (33%). Colonoscopy was performed in 6 patients and esophagogastroduodenoscopy was performed in 2 patients. Bronchoscopy was performed in 1 patient who was diagnosed with endobronchial tuberculosis. PET was performed in 8 patients (8%), and in this group, definitive diagnosis was obtained in 7 patients; there were 3 patients with malignancies, 3 with CTD, 1 with necrotizing lymphadenitis, and 1 patient who remained undiagnosed and recovered.
Sixty-five patients (65%) were treated with antibiotics at the time of admission for FUO evaluation. Among these, only 4 patients (6.2%) were diagnosed with bacterial infection. During their hospitalization, 70 patients (70.0%) received antibiotics, 22 patients (22.0%) received steroid therapy, 63 patients (63.0%) received nonsteroidal anti-inflammatory drugs (NSAIDs), and 5 patients (5.0%) did not receive any treatments. Of the 19 patients with infectious diseases, 16 patients (84.2%) received antibiotics treatment. Of the 15 patients with CTD, 13 patients (86.7%) received NSAIDs and 9 patients (60.0%) received steroid therapy.
All of the patients who were diagnosed with necrotizing lymphadenitis received NSAIDs and excisional biopsy. Among these, 2 patients had received steroid therapy and 2 patients received intravenous immunoglobulin. These patients who received steroid therapy and intravenous immunoglobulin had relapsing fever that was only controlled after excisional biopsy.
Ninety-two patients (92%) became afebrile during their hospitalizations. Eight patients (8.0%) had persistent fever until discharge, including 2 patients with JIA, 1 patient with necrotizing lymphadenitis, 1 patient with mycoplasma pneumonia, and 4 undiagnosed patients. One undiagnosed patient was lost to follow-up after discharge and the other 7 patients became afebrile during the outpatient follow-up period. Eleven patients (11.0%) received treatment in the intensive care unit; 2 had HLH, 2 had JIA, 2 had infective endocarditis, 2 had acute demyelinating encephalomyelitis, 1 had septic arthritis, and 2 were undiagnosed.
One patient (1%) with HLH died 10 days after the diagnosis during the hospitalization. There were no cases of mortality in the undiagnosed patients.
Discussion
This study investigated the etiology and clinical characteristics of pediatric FUO patients from a single center in Korea for 15 consecutive years from 2000 to 2014. The present study showed that no diagnosis (43%) was the most common etiology. Among the confirmatory diagnoses, infectious disease (19%) was most common followed by CTD (15%).
In many studies, infectious diseases have been the most common cause of FUO. Chow and Robinson2) summarized 18 studies on pediatric FUO in a systematic review in 2011. Eight studies were performed in developed countries (USA, Spain, and Germany) and published from 1970 to 1998. The other 10 studies were performed in developing countries and published from 1994 to 2008. In sum, infectious disease (51%) was the most common cause, although 22% of cases were undiagnosed. Among the ten studies in developing countries, infectious disease was the most common etiology (36% to 78%). Among the eight studies in developed countries, infectious disease was the most common etiology in 6 studies and no diagnosis was most common etiology in 2 studies that were performed in the United States67). Similarly, in a previous Korean study from 1999 to 20043), infectious disease was also the most common etiology (41.7%), and 27.5% of patients were undiagnosed. Of note, our study showed that undiagnosed patients made up the greatest proportion of FUO cases. Recent studies have revealed that the proportion of undiagnosed cases has increased over time due to improved diagnostic technique. It is thought that improved techniques make it easier to diagnose certain diseases earlier before the patient meets criteria for FUO348).
Echocardiography, ultrasonography, CT, and MRI are performed as basic diagnostic evaluation for FUO. Unfortunately, these standard approaches generally exhibit a relative low sensitivity and specificity9). PET-CT has been used in some studies for the diagnosis and management of FUO, owing to its ability to assess the morphology and functional characteristics of the tissues simultaneously910). In Tokmak et al.9)'s study reported that the estimated diagnostic accuracy, sensitivity and specificity of PET-CT for FUO were 90.5%, 93.8% and 80%, respectively. However, PET-CT has several disadvantages include high radiation doses, relatively high cost, limited availability, high rate of false-positive results and its inability to detect systemic, nonfocal disease11). Although PET-CT can be used as a valuable tool for evaluation of certain cases of FUO, clinicians should consider both advantages and disadvantages of PET-CT and use this modality in limited situations. Another diagnostic technique, sequencing of the 16s ribosomal RNA (rRNA) gene, has been successfully used for bacterial identification in culture negative samples where there is a clinical suspicion of microbial involvement. The 16s rRNA sequencing has higher culture rate of bacteria than traditional culture methods and novel species can be detected12).
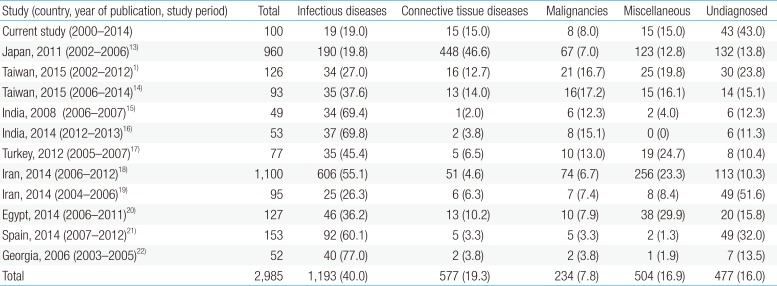
Since the development of diagnostics has been rapidly evolving in many areas of medicine, including infectious diseases, it may not be accurate to combine data from earlier years with data after 2000, when more advanced diagnostic methods became available in clinical practice. Therefore, in Table 6, we only reviewed the data between 2000 and 2014 from various countries113141516171819202122). Although infectious disease still appears to be the most common cause of FUO in children, it would be interesting to assess for changes in the etiologies of FUO in the future.
Many infectious diseases have been reported to be causes of FUO in children1314161719). In Chow and Robinson2)'s systematic review, bacterial infections (59% of all infections) were the most common, and viral infection made up only 7% of all infections. Among viral infections, Epstein-Barr virus was the most common viral pathogen, which was similar to the results of Ikhlas et al.16)'s study showing that a viral etiology made up 8.1% of the infectious diseases group. In our study, 19 patients (19%) had infectious diseases with viral infection being the most common (n=8, 40%). Epstein-Barr virus was identified in 2 patients. Of note, respiratory viruses were the most common cause of infectious disease in our study. The causative viruses of respiratory infection were RSV, adenovirus, and parainfluenza virus. All of these viruses were detected by culture in 2001, 2005, and 2008 before PCR was available in our institution. Currently, we perform multiplex respiratory virus PCR, which allows us to diagnose any respiratory viruses within 24–48 hours. Therefore, the number of patients with FUO caused by respiratory virus infection is expected to decrease over time. In addition, we cannot rule out the possibility that those respiratory viruses were nosocomial infection since three out of 4 patients had been hospitalized in an outside hospital before being referred to our hospital for evaluation for FUO.
M. tuberculosis was detected in three patients (15.8% of the cases of infectious disease), which was similar to the results from previous studies in countries with a high prevalence of tuberculosis including Korea, China, Iran, and India1618). Chow and Robinson2) concluded that there was difference in the types of infections responsible for pediatric FUO between developing and developed countries. Bartonella infection was more common in developed countries, while brucellosis, typhoid fever, and tuberculosis were more common in developing countries. Many studies emphasized that tuberculosis should be considered to be a cause of FUO in endemic countries2324). In a study published in 2006 by Park et al.3), 8 patients (21% of cases of FUO caused by infectious disease) were diagnosed with tuberculosis infection. In contrast to Park et al.3)'s study, in which seven of eight patients had positive TST, all three patients with confirmed tuberculosis infection in our study had negative TST but positive culture results. Therefore, even when the TST result is negative, culture and PCR should be performed when there is a suspicion for tuberculosis.
CTD is the second most common cause of FUO in many studies 20). Among these, JIA was most common, followed by SLE, as in our study21420). All of our 15 patients (15%) had JIA or SLE, while various CTD such as autoimmune cholangitis, polyarthritis nodosa, and rheumatic fever have been reported in another study25). It is well-known that CTD is more common in girls. In our study, nine of the patients (60%, 9 of 15) with CTD were girls while Cho et al.1) reported that all of the patients diagnosed with SLE were girls. ANA and anti-ds DNA are useful diagnostic markers for CTD. In our study, 6 patients (40% of the CTD cases) had a positive ANA. Among them, 3 patients (20% of the CTD cases) with SLE had positive ANA and anti-ds DNA.
In our study, the number of patients with a diagnosis of malignancy was 7 (7%, 7 of 100), of which three had lymphoma and two had neuroblastoma. Although the proportion of patients with leukemia has been high in other studies114171920), there were no cases of leukemia in our study. It is thought that leukemia is diagnosed earlier by early bone marrow examination when cytopenia or blasts are seen in complete blood cell counts or peripheral blood smears. Since malignancy is the main cause of death in FUO patients, early diagnosis is important to reduce mortality. Therefore, appropriate imaging studies or early invasive procedures such as bone marrow examination should be performed in certain patients with suspicious presentation for malignancies.
Necrotizing lymphadenitis, also known as Kikuchi-Fujimoto disease, is common in young Asian women and has seen recent increases in its prevalence in children3). Eight patients (8%) were diagnosed with necrotizing lymphadenitis in our study and the frequency was increased compared with a previous study published 10 years earlier in Korea3). In patients with enlarged lymph nodes accompanied with fever, the possibility of necrotizing lymphadenitis should be considered. In a Japanese study13), 14 patients with FUO had necrotizing lymphadenitis, which was the most common diagnosis in 34 patients in the miscellaneous group.
Regarding the duration of fever, except undiagnosed etiology, our study showed that the percentage of CTD was highest in patients who had fever>28 days and the percentage of infectious disease was highest in patients who had fever≤28 days. In another pediatric study, there was no difference in the frequencies of CTD in patients with fever 14–30 days and in patients with fever over 30 days1). In one study of adults, there were no significant relationship between fever duration and the etiology of FUO26). In another study of adults, Yu et al.27) reported that the infection group had a relatively shorter average duration of fever than the other groups.
A major difference between adult and pediatric FUO is prognosis. The prognosis of pediatric FUO is good compared to FUO in the adult due to differences in etiologies. Even if pediatric FUO remains undiagnosed, many cases resolve spontaneously8). In our study, 92 patients (92%) were no longer febrile by the time of discharge, but almost all patients who had persistent fever eventually improved after discharge. Only 1 patient (1%), who was diagnosed with HLH, died despite receiving appropriate chemotherapy. In another study, Cho et al.1) reported that 9 of 126 patients (7.14%) died, and none of the patients who remained undiagnosed had unfavorable outcomes. Four of 91 patients (4.4%) died in Park et al.'3)s study; 1 patient had HLH, 1 had acute respiratory failure after adenoviral infection, and 2 had been not diagnosed. The original studies on pediatric FUO from the 1970s reported a mortality rate of 6% to 9%4), but the etiologies and mortality rates may have changed since then. Therefore, further study is needed to understand the trends in mortality and overall outcomes associated with pediatric FUO.
There are some limitations in our study. First, because of the study's retrospective nature, the records were not thorough, especially the physical examination and the patients' signs and symptoms. Second, our center is a tertiary-care hospital and we only included inpatient cases for this analysis. Therefore, there is a chance of selection bias. However, we believe that this study provides meaningful contributions to our knowledge of pediatric FUO.
In conclusion, the most common etiology of pediatric FUO in our study is no diagnosis, followed by infectious diseases. Mortality was observed in 1% of patients and appears to be lower than in previous reports.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.