Clinical and laboratory findings of childhood buckwheat allergy in a single tertiary hospital
Article information
Abstract
Purpose
Buckwheat allergy is one of the most severe types of food allergy in some countries, especially among children. However, few studies have investigated this condition. The aim of this study was to report the clinical and laboratory findings in Korean children with buckwheat allergy.
Methods
Thirty-seven subjects, aged 1 to 14 years, were enrolled by retrospective medical record review from January 2000 through May 2015 at the Department of Pediatrics in Ajou University Hospital. The demographic profile, previous exposure to buckwheat pillows, clinical symptoms, and laboratory findings of each subject were recorded.
Results
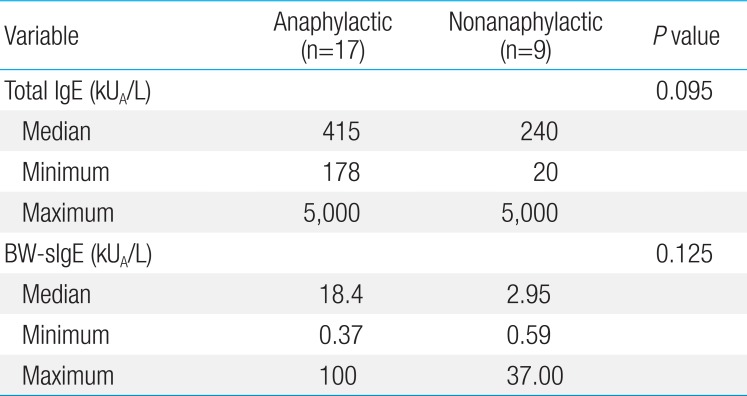
Twenty-six of the 37 children had immediate-type allergic symptoms to buckwheat, while 11 subjects were tolerant to buckwheat. Seventeen out of 26 buckwheat allergic children (65.4%) had anaphylaxis. The median buckwheat specific IgE level in the buckwheat allergic group (7.71 kUA/L) was significantly higher (P<0.001) than in the buckwheat tolerant group (0.08 kUA/L) with an optimal cutoff value of 1.27 kUA/L (sensitivity 84.6%, specificity 100%). When adjusted for age, the difference between the 2 groups showed no statistical significance (P=0.063). In subjects who had anaphylaxis, buckwheat-specific IgE levels ranged from 0.37 to 100 kUA/L.
Conclusion
Almost two-thirds of buckwheat-allergic children had anaphylaxis, and a wide-range of buckwheat specific IgE levels were observed in these children. Anaphylaxis occurred in a subject with a remarkably low IgE level (0.37 kUA/L).
Introduction
Buckwheat (BW) (Fagopyrum esculentum), which belongs to the Polygonaceae family, is an important ingredient in various traditional Asian dishes. It is also used to make gluten-free flour that can be consumed by patients with celiac disease1). BW allergy is a typical IgE mediated type 1 hypersensitivity, which is a major cause of anaphylaxis1). A 1998 Japanese study with 92,680 subjects reported that 0.21% (194 children) of school-aged children had a BW allergy2). In Korea, the prevalence of BW allergy was 0.1% (in elementary school students) and 0.13% (middle school), and BW was the fifth leading cause of anaphylaxis in children from a recent multicenter study at 23 tertiary hospitals34). In Europe, BW allergy caused food-related anaphylaxis in 4.5% of adults in France and 1.7% of adults in Italy who visited allergy clinics56).
In Korea and Japan, BW husk pillows are commonly used, but studies investigating the relationship between exposure to BW husk pillows and BW allergy are rare. Two case reports document immune events related to using BW husk pillows. In 1 case report, 3 patients had chronic asthma due to exposure to BW husk pillows, and in another case report, 2 patients were sensitized to BW after using BW husk pillows78).
The major allergens of BW are 8–10 kDa, 16 kDa, 19 kDa, and 24 kDa proteins; the 19 kDa protein is relatively specific for clinical reactions to BW9). More recently, the 40–50 kDa protein is implicated as an important allergen to predict moderate to severe clinical symptoms in Korean children with BW allergy10). BW is known to have cross-reactivity with latex, rice, poppy seeds, and quinoa11121314).
The relationship between the level of buckwheat-specific IgE (BW-sIgE) and clinical symptoms to BW has not been substantially studied. A 2003 report demonstrated that a BW-sIgE level of 1.26 kUA/L had a positive predictive value of 79.75% in predicting BW allergy in children15). The measurement of BW-sIgE has limitations in the diagnosis of a BW allergy, and is not a definitive predictor of symptom severity. The aim of this study was to report the clinical and laboratory findings of BW allergy in Korean children.
Materials and methods
1. Subjects
A retrospective medical record review was done for children who visited the Department of Pediatrics at Ajou University Hospital for suspicious food allergies from January 2000 through May 2015. Thirty-seven children who underwent BW-sIgE assay with history of ingestion of BW were enrolled. The BW-allergic group (n=26) comprised children who had immediate-type allergic reactions after ingesting BW, and the BW-tolerant group (n=11) comprised children who had no clinical reactions after ingesting BW. The BW-allergic group was further classified into 2 subgroups according to symptoms: anaphylaxis group and nonanaphylaxis group. The anaphylaxis group comprised children with a history of anaphylaxis after ingesting BW. Anaphylaxis was defined according to the National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network16). The nonanaphylaxis group comprised children who experienced acute allergic reactions such as urticaria, but not anaphylaxis after ingesting BW. The study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-MED-MDB- 15-171).
2. Measurement of the total IgE and specific IgE antibody levels
Serum concentrations of total IgE and BW-sIgE for all subjects were measured by means of ImmunoCAP, according to the manufacturer's instructions (Thermo Fisher Scientific, Uppsala, Sweden). The lower and upper limits of BW-sIgE were 0.05 and 100 kUA/L, respectively.
3. Statistical analysis
The Mann-Whitney U test was used to compare characteristics and serologic parameters between study groups. Receiver operating characteristic (ROC) curves were constructed for total IgE and BW-sIgE.
Results
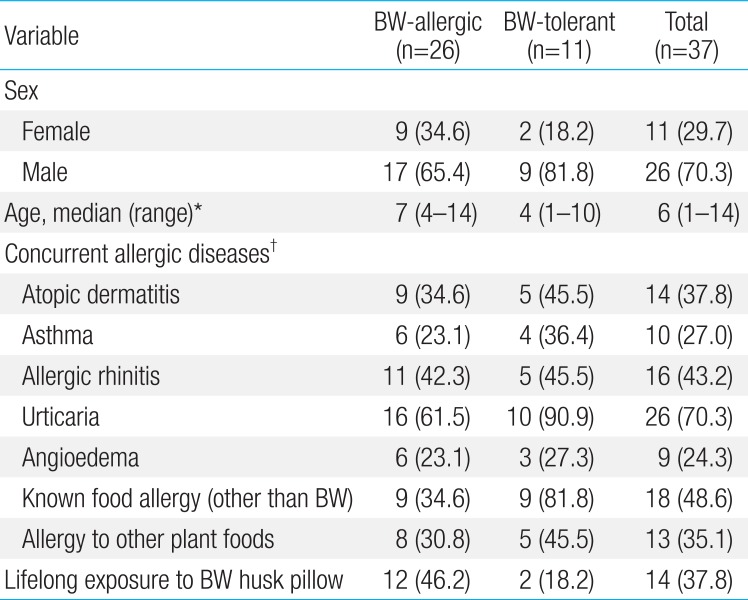
All 37 subjects were included in the study analysis. The BW-allergic group (n=26) consisted of 17 males and 9 females, with a median age of 7 years, ranging from 4 to 14 years. Seventeen subjects in the anaphylaxis group experienced anaphylaxis after ingesting BW, and 9 subjects in the nonanaphylaxis group had acute allergic reactions such as urticaria, angioedema, and/or vomiting after ingesting BW, but not anaphylaxis. Eleven subjects in the BW-tolerant group had no immediate-type allergic symptoms after ingesting BW. In the BW-allergic group, 9 (34.6%) had other previously known food allergies, mostly to plant foods such as wheat (Table 1).
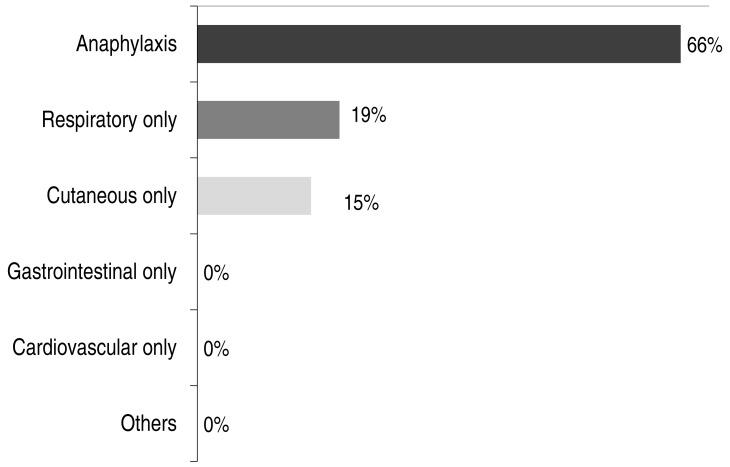
In terms of clinical manifestations, 17 children (66%) in the BW-allergic group experienced anaphylaxis, 19% had respiratory symptoms only, and 15% had cutaneous symptoms only. None of the subjects had gastrointestinal or cardiovascular symptoms only (Fig. 1).
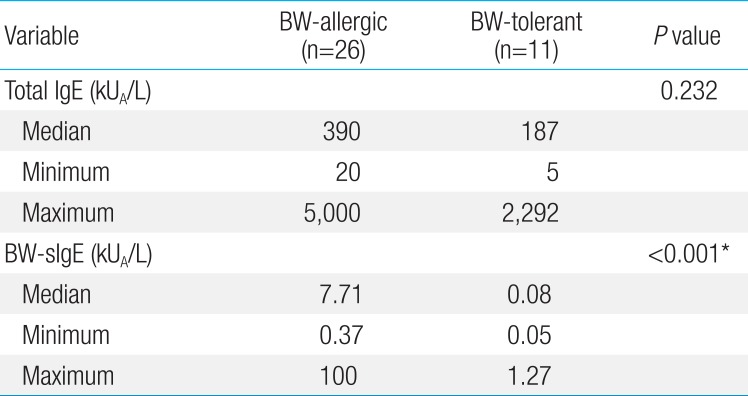
The median level of total IgE in the BW-allergic group was 390 kUA/L, which was higher than that of the BW-tolerant group (187 kUA/L) but this difference was not statistically significant (P=0.232) (Table 2). The median level of BW sIgE in the BW-allergic group was 7.71 kUA/L, which was significantly higher (P<0.001) than that of the BW-tolerant group (0.08 kUA/L) before age adjustment. The difference in the levels of BW-sIgE between BW-allergic and BW-tolerant groups showed no statistical significance (P=0.063) when adjusted for age by linear regression analysis. The median level of BW-sIgE in the anaphylaxis group was 18.4 kUA/L, which was higher than that of the nonanaphylaxis group (2.95 kUA/L) but this difference was not statistically significant (P=0.125) (Table 3). In addition, the median BW-sIgE levels were compared between the BW-tolerant group and anaphylaxis group, the level was significantly higher in the anaphylaxis group even after age adjustment (P=0.021, data not shown).
To differentiate patients who experienced immediate-type allergic reactions after ingesting BW from those who were tolerant to BW, the optimal cutoff values for baseline concentrations of total IgE and BW-sIgE were obtained from the ROC curves (Fig. 2). To maximize both sensitivity and specificity at the same time, the optimal cutoff value of BW-sIgE to predict clinical reactivity to BW was >1.27 kUA/L, with a sensitivity of 84.6% and a specificity of 100%.
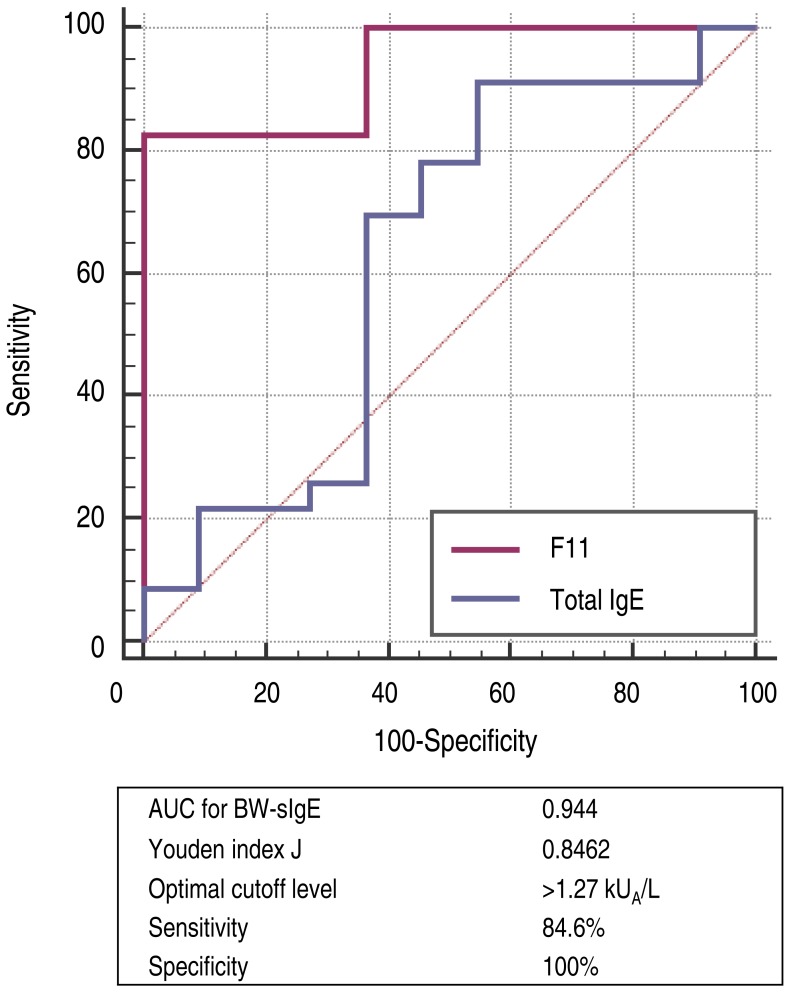
Receiver operating characteristic curve (BW-allergic vs. BW-tolerant). BW, buckwheat; BW-sIgE, buckwheat specific immunoglobulin E; AUC, area under the curve.
Fourteen subjects had a history of exposure to BW husk pillows. However, the causal relationship between BW husk pillows and onset of symptoms after exposure to BW could not be identified due to the retrospective design of the study. The percentages of subjects with a history of exposure to BW husk pillows in the BW-allergic group and BW-tolerant group were 46.2% and 18.2 %, respectively (Fig. 3). Additionally, half of patients who had respiratory symptoms after ingesting BW had a history of exposure to BW husk pillows, while only 28.6% of patients who had no respiratory symptoms after ingesting BW used BW husk pillows in the past.
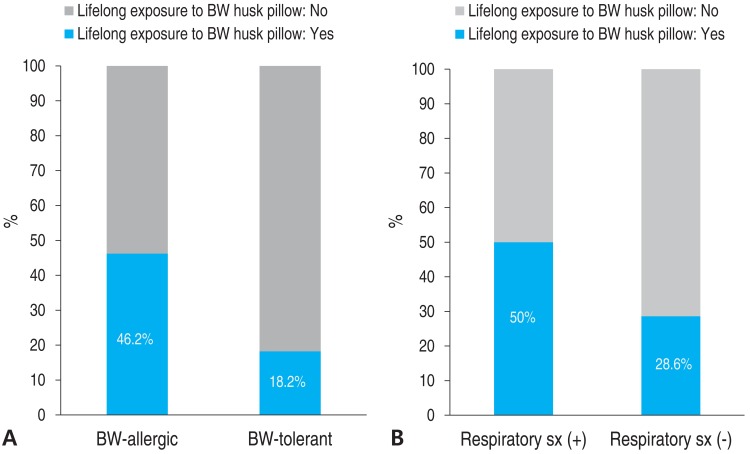
(A) BW allergy and lifelong exposure to BW husk pillows. (B) Respiratory symptom (sx) after BW ingestion and lifelong exposure to BW husk pillows. BW, buckwheat; sx, symptom.
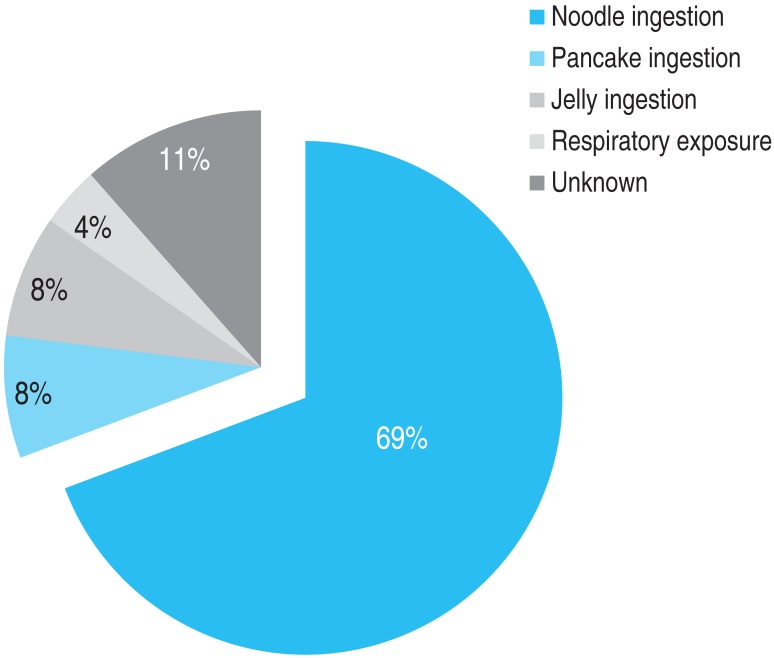
The frequency of the type of BW exposure was the following: noodle ingestion in 18 subjects (69%), pancake ingestion in 2 subjects (8%), jelly (muk) ingestion in 2 subjects (8%), respiratory exposure in 1 subject (4%), and unknown in 3 subjects (11%) (Fig. 4).
Discussion
BW is a common cause of anaphylaxis in Asian countries. In one Japanese study, BW was the seventh leading cause of allergen-related anaphylaxis across all ages17). The prevalence of BW allergy among Korean and Japanese school-aged children was 0.36% and 0.21%, respectively317). BW allergy is a growing concern in Western countries as the consumption of BW increases, but few studies have been reported. We focused on the clinical details of childhood BW allergy and the relationship between BW-sIgE concentration in BW-allergic subjects.
In this study, the median age of BW-allergic children was 7 years, ranging from 4 to 14 years. This finding corresponds with an earlier report with a mean age of 8.34±0.51 years, ranging from 4 to 14 years15). In addition, as in previous pediatric study, a male predominance was observed in our study15). Nine out of 26 children in the BW-allergic group had other previously known food allergies, most of them being plant foods. From previous studies, BW is known to have cross-reactivity with latex, rice, poppy seeds, and quinoa11121314). However, our subjects who had previous plant food allergies were allergic to other plant sources, specifically, wheat (7 out of 8) and mung beans (1 out of 8) which are not known to have cross-reactivity with BW thus far.
The proportion of subjects who had a history of using BW husk pillows was higher in the BW-allergic group than in the BW-tolerant group. Subjects who had respiratory symptoms used BW husk pillows more often compared with those who did not have respiratory symptoms. One case report suggested that a small amount of BW flour attached to a BW husk pillow could induce sensitization and cause nocturnal asthma7). A case report in 2009 from the United States suggested that initial ingestion of BW food caused anaphylaxis after previous sensitization by BW husk pillow8). Our data suggests that some subjects have been sensitized by using BW husk pillows, but since the timing of pillow usage and ingestion of BW were not recorded, it is difficult to explain causal relationships.
In a previous analysis, 13% of patients who had anaphylaxis were counted among patients with food allergies in the United States, 23% in Japan, and 7.6% of patients below the age of 6 years in Korea171819). From another study on pediatric peanut allergy in Canada, anaphylaxis was found in 36.7%20). Within patients who were sensitized to BW, 29.2% had anaphylaxis in Italy21). In the present study, 66% of BW-allergic patients had anaphylaxis, which was remarkably higher than in previous studies. Patients with mild allergic reactions may go to local clinics, whereas patients with more severe symptoms may go to university hospitals. In this study, data were collected from a university hospital, which may explain why we noted a higher proportion of anaphylaxis patients compared to previous studies. This may also contribute to bias. The frequencies of respiratory (19%) and cutaneous (15%) symptoms were comparable with findings of earlier studies in both adults and children, whereas frequencies of gastrointestinal symptoms were relatively lower in this study151921).
Reports on BW-sIgE levels in BW allergy are rare. In previous studies, mean BW-sIgE was 6.23 kUA/L in patients with suspected BW allergy who were older than age 17, and median 29.23 kUA/L in children 1521). However, the BW-sIgE levels between the allergic and nonallergic groups had no significant differences. In the present study, the BW-allergic group had a higher level of median BW-sIgE than in the BW-tolerant group but the difference showed no statistical significance when adjusted for age between the groups. From the ROC curves, the optimal cutoff value of BW-sIgE to differentiate the BW-allergic group from the BW-tolerant group in this study was 1.27 kUA/L with a sensitivity of 84.6% and a specificity of 100%. A previous study reported the optimal cutoff level was 1.26 kUA/L, with a sensitivity of 93.1% and a specificity of 93.33%, which coincides with the result of this study15). The median BW-sIgE level was higher in the anaphylaxis group than in the nonanaphylaxis group but this difference was not statistically significant (P=0.125). As the range of BW-sIgE levels in the anaphylaxis group was wide (0.37 to 100 kUA/L), it may be suggested that severe allergic reactions to BW could be provoked even at a very low BW-sIgE levels. On the other hand, when the median BW-sIgE levels were compared between the BW-tolerant group and anaphylaxis group, the level was significantly higher in the anaphylaxis group even after age adjustment (P=0.021, data not shown).
There were several limitations in this study. First, the data from retrospective studies may be less complete compared to data from prospective studies because of partial or lack of documentation. In patients with very low levels of BW-sIgE, an oral food challenge test should have been performed to confirm the causal relationship but it was not possible in this study due to the retrospective nature. The amount and form of BW may have differed among each patient, and the timing of exposure to BW husk pillows and the first ingestion of BW could not be specified from the retrospective medical record review. Second, the total number of subjects was relatively small, when compared to studies of other food allergies. However, it is compatible with previous studies of BW allergy considering low prevalence, especially in children. Finally, our findings are not representative for other populations due to geographical and cultural differences. In the future, it is necessary to do a prospective study with a larger population.
To conclude, the findings of this study suggests that BW caused a severe form of allergic reaction, such as anaphylaxis, in almost two-thirds of BW-allergic children, and the optimal cutoff level of BW-sIgE to differentiate BW-allergic children from BW-tolerant children was 1.27 kUA/L.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.