Clinical significance of serum alanine aminotransferase and lifestyle intervention in children with nonalcoholic fatty liver disease
Article information
Abstract
Purpose
This study aimed to investigate the clinical significance of serum alanine aminotransferase (ALT) levels in children with nonalcoholic fatty liver disease (NAFLD) and the effect of lifestyle intervention on NAFLD.
Methods
The clinical data of 86 children diagnosed with NAFLD were reviewed retrospectively. Forty-six patients belonged to the elevated ALT group and 40 to the normal ALT group. The clinical parameters of patients with NAFLD were also compared based on the status of ALT levels after lifestyle intervention.
Results
Patients with elevated ALT had significantly higher body mass index (BMI) scores than those with normal ALT (P<0.05). Of all the patients with elevated ALT, 89% exhibited moderate or severe degree of fatty change in the liver on ultrasonographic examination, whereas most patients with normal ALT exhibited mild or moderate degree changes. Liver biopsy was performed in 15 children with elevated ALT and all showed mild histological changes. Of all patients with elevated ALT, 49% achieved normal ALT levels after lifestyle intervention. Those with more severe histological changes tended to have continuously increasing ALT levels. There was no correlation between the normalization of posttreatment ALT level and BMI, as well as ultrasonographic findings at diagnosis.
Conclusion
ALT elevation in NAFLD is highly associated with higher BMI scores and more severe degree of fatty changes on ultrasonographic examination. Lifestyle intervention can significantly improve ALT in children with NAFLD. The degree of histologic changes appears to be a predictor of the treatment response to NAFLD.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a medical condition ranging from simple liver fat accumulation (simple steatosis, SS) to fat accumulation plus necroinflammatory activity with or without fibrosis (nonalcoholic steatohepatitis, NASH). NAFLD is the most common cause of liver disease in the preadolescent and adolescent age groups in the United States1). While the natural history of NAFLD remains incompletely understood, NASH has potential to lead to cirrhosis and hepatocellular carcinoma23). No clinical features, biochemical parameters, or imaging studies have been identified that allow for reliable distinction between various forms of NAFLD4).
The degree of fatty involvement of the liver may provide a marker for estimation of the presence and severity of the disease56). Imaging studies such as ultrasonography, computed tomography, and magnetic resonance imaging are often used in assessing NAFLD, but liver biopsy is required to differentiate between NASH and SS. However, liver biopsy is invasive and is not frequently performed in the pediatric population47).
Liver function tests are used to determine the presence of hepatic damage or impaired function. The suspicion of NASH is usually prompted by abnormal elevated alanine aminotransferase (ALT). NAFLD is the most common cause of abnormal liver test among adults with a prevalence of 13%–23%, and it represents a key emerging clinical problem affecting a substantial proportion of children and adolescents (2.6%–9.8%), especially in the presence of overweight/obesity (>77%)189). In clinical studies, elevated ALT was found in 24%–25% of overweight/obese children1011). Elevated liver enzyme levels, particularly unexplained ALT elevation, have been widely used as surrogate markers for NAFLD1213).
There are several reports regarding clinical correlates of liver histology features in pediatric NAFLD. Schwimmer et al.14) showed that liver pathology in children with NAFLD is associated with insulin resistance and increased serum ALT. Jee et al.15) reported that serum triglyceride levels and ultrasonographic findings are highly correlated with pathologic findings in children with NAFLD. However, no consensus exists as to whether serum aminotransferase values correlate with histologic features.
There are few studies on the effect of therapeutic intervention with lifestyle modification on liver disease in children. Several studies reported that lifestyle intervention (dietary restriction and exercise) has improved the liver function of patients with NAFLD 1617).
The aim of this study was to better characterize the presentation of NAFLD and to investigate the clinical significance of ALT elevation in children with NAFLD. The authors also sought to determine the effect of lifestyle intervention with diet and physical exercise in children with NAFLD. The predicting factors of treatment response of NAFLD were evaluated by comparing the clinical and histological findings between a group whose ALT level was normalized after lifestyle intervention and a group whose ALT level increased continuously.
Materials and methods
1. Subjects and diagnostic criteria of NAFLD
Eighty-six patients who visited the Department of Pediatrics, Pusan National University Hospital because of obesity or abnormal liver function test between January 1994 and August 2007 and who were diagnosed with NAFLD were included in this study. Subjects who showed steatosis on ultrasonography with or without elevated serum ALT (>40 U/L) were diagnosed as having NAFLD14).
We excluded patients who had metabolic hepatitis, toxic hepatitis, viral hepatitis, congenital metabolic disease or Wilson disease, autoimmune hepatitis, hepatobiliary anomaly, or other diseases that might affect the liver enzyme level.
2. Analysis of medical records
The clinical characteristics such as gender, age, body mass index (BMI), triglyceride, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were analyzed retrospectively in patients with NAFLD. A follow-up study after treatment was possible in 37 patients with ALT elevation. The clinical and liver histological findings were compared between patients according to ALT status.
3. Degree of steatosis on ultrasonography
The criterion for steatosis was hyperechogenic liver tissue with fine, tightly packed echoes. The degree of steatosis was assessed by the fall in echo amplitude with depth (rate of posterior beam attenuation), increasing discrepancy of echo amplitude between liver and kidney, and loss of echoes from the walls of the portal veins18). Mild steatosis was recognized by a slight increase in liver echogenicity, a slight exaggeration of liver and kidney echo discrepancy, and relative preservation of echoes from the walls of the portal vein. Moderate steatosis was accompanied by loss of echoes from the walls of the portal veins, particularly from the peripheral branches, resulting in a featureless appearance of the liver. In addition, greater posterior beam attenuation was found and a greater discrepancy between hepatic and renal echoes. Severe steatosis was recognized by a greater reduction in beam penetration, loss of echoes from most of the portal vein wall, including the main branches, and a large discrepancy between hepatic and renal echoes.
4. Grading the histological lesions of steatohepatitis
Liver biopsy was conducted in 15 patients with elevated ALT and increased echogenicity on liver ultrasonography. All patients were diagnosed as having biopsy confirmed steatohepatitis. The severity of inflammation in the portal area, lobular inflammatory activity, and fibrosis were classified based on the necroinflammatory grading system for steatohepatitis by Brunt et al.19).
5. Identification of predicting factors of treatment response
Lifestyle intervention was applied to the patients with ALT elevation, and measurement of body weight and blood tests were performed periodically. A low-calorie diet and regular aerobic exercise for over 30 minutes every day were recommended. We divided 12 patients in which a follow-up study was possible after a year into a group with normal serum ALT and a group with continuously elevated ALT. Histological findings of the liver between the 2 groups were compared.
The data were analyzed using the SPSS 12.0 ver. (SPSS Inc., Chicago, IL, USA). Student t test was used to compare gender distribution, BMI and biochemical findings between patients with or without ALT elevation, and to compare the biochemical and histological findings in patients with NASH according to ALT status after lifestyle intervention. Mantel-Haenszel chi-square test was used to compare age distribution and the severity of steatosis on ultrasonography. Statistical significance was accepted if P<0.05.
Results
1. Gender and age distribution of the subjects
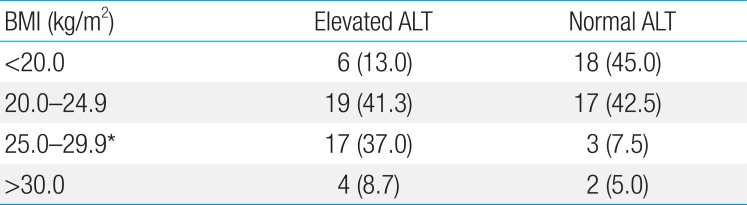
Among 86 patients, elevation of serum ALT was present in 46 (53.5%). The male/female ratio was 3.6:1 in elevated ALT group and 1.7:1 in normal ALT group. The number of male was higher in both groups, but the difference was not statistically significant (Table 1). Overall mean age was 8.6±3.8 years, 9.7 years in elevated ALT group, and 7.2 years in normal ALT group. Age distribution of patients was as follows: 10.8% of patients in elevated ALT group and 30.0% of patients in normal ALT group were 5 years old or younger, 45.9% and 50.0% were 6–10 years, and 41.3 %, and 20.0% were 11 years old or older, respectively. Age distribution was not significantly different between the 2 groups, but patients with normal ALT tended to be younger, and patients with elevated ALT to be older (Table 1).
2. Clinical and biochemical findings
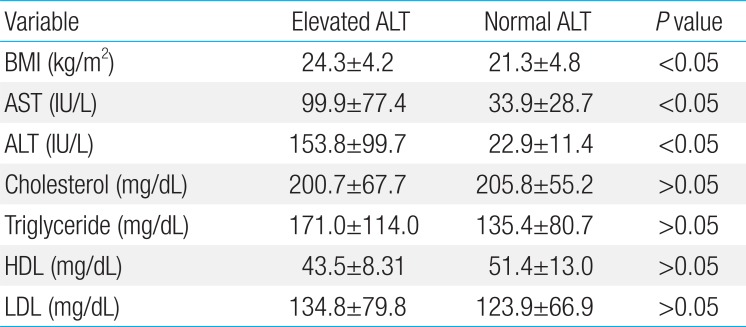
BMI was 24.3±4.2 kg/m2 in elevated ALT group and 21.3±4.8 kg/m2 in normal ALT group. There was a significant difference between the 2 groups (P<0.05). The proportion of patients whose BMI was over 25 kg/m2 was 45.7% in elevated ALT group, significantly higher than 12.5% in normal ALT group (P<0.05) (Table 2). Serum AST and ALT levels were 99.9±77.4 and 153.8±99.7 IU/L, respectively, in elevated ALT group and 33.9±28.7 and 22.9±11.4 IU/L in normal ALT group. There was a significant difference between 2 groups (P<0.05). However, serum cholesterol, triglyceride, HDL-C, and LDL-C levels were not significantly different between the 2 groups (Table 3).
3. Degree of steatosis on ultrasonography
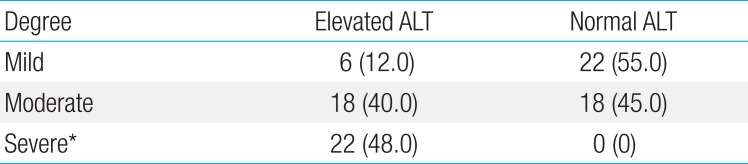
On ultrasonography, 47.8% of patients with ALT elevation showed severe degree steatosis and 12% showed mild degree. None of patients with normal ALT showed severe degree steatosis and 55.0% showed mild degree. Thus, severe steatosis was significantly more frequent in patients with ALT elevation (P<0.001) (Table 4).
4. Comparison of the clinical parameters of patients with elevated ALT according to the status of ALT levels after lifestyle intervention
Among 37 patients with ALT elevation who had been followed up at least a year with lifestyle modification, 18 (48.6%) showed normal serum ALT levels, and 19 (51.4%) showed continuously increasing ALT levels. The 2 groups were not different in BMI, serum AST, ALT, cholesterol, triglyceride, HDL-C, and LDL-C levels (Table 5). Degree of fatty change on ultrasonography showed no significant difference before and after treatment in both groups.

Comparison of initial clinical and biochemical characteristics between normalized and continuously elevated ALT level after treatment in patients with elevated ALT
Among the patients with ALT elevation, 15 underwent liver biopsy before treatment. Overall, the findings of inflammation in and around the portal vein, lobular inflammatory necrosis, and fibrosis were mild (Table 6). Twelve patients with ALT elevation whose follow-up was available for a year were divided into 2 groups, a group with normalized ALT and a group with continuously increasing ALT. When their liver biopsy findings were compared, those with more severe histological changes tended to have continuously increasing ALT, though the difference was not statistically significant (Table 6). All the patients with normalized ALT showed none portal and periportal inflammation, and grade 1 lobular inflammation. However, those who have continuously increasing ALT after treatment showed mild (70%) portal and severe (10%) periportal inflammation, grade 2 lobular inflammation (20%). The finding of fibrosis was similar in both groups.
Discussion
This study revealed that BMI was significantly higher in the patients with elevated ALT than in those with normal ALT. And the degree of hepatic steatosis on ultrasonographic examination is significantly higher in patients with elevated ALT than those with normal ALT. Those results suggest that severity of obesity is closely related to ALT elevation and the degree of fatty change in the liver.
ALT is found primarily in the liver and considered one of the indicators of hepatocellular health. Burgert et al.20) reported that deterioration in glucose and insulin metabolism might emerge as a function of increasing ALT elevation and hepatic fat accumulation.
In the absence of liver biopsy, presumed NAFLD is diagnosed by ultrasonographic hepatic appearance and/or elevated serum ALT21). Previous reports argued that mild to moderate elevation of ALT is present in 70%–100% of patients with NASH2223). NAFLD can also occur in subjects with normal ALT. Fracanzani et al.24) found that 14% of patients among 458 adults with biopsy confirmed NAFLD have normal levels of ALT. However, there is no significant correlation between the degree of serum ALT elevation and the histologic features2225).
In this study, mean age of the patients was 8.6 years, which means that NAFLD can occur at a young age in Korea. The age of patients with normal ALT was inclined to be young, and that of patients with elevated ALT was old. Ko et al.26) reported that children with NASH were older than those with SS.
With respect to the prevalence of NAFLD, Schwimmer et al.27) reported boys had the higher prevalence of NAFLD (44%) than girls using ALT as a surrogate of NAFLD. Schwimmer et al.6) also reported boys had the higher prevalence of NAFLD (7.4%) than girls (0.5%) using liver biopsy. In our study, no statistically significant difference in prevalence of NAFLD between men and women was observed, although the proportion of boys was higher in both the elevated ALT group and the normal ALT group.
Dyslipidemia, especially elevated triglycerides is a primary risk factor for NAFLD and is also related with outcome of NAFLD828). Yoo et al.13) reported that the high levels of triglycerides and total cholesterol, low levels of HDL-cholesterol were significantly associated with elevated ALT (NAFLD) in obese Korean children. In our study, levels of serum cholesterol, triglyceride, LDL-C, and HDL-C were not significantly different between the elevated ALT group and the normal ALT group.
The sensitivity of ultrasonography for diagnosing hepatic steatosis was reported to be 85%–90%29). Ultrasonography is considered to be highly sensitive and consistent with biopsy results in determining hepatic fatty infiltration and the severity of steatosis 530). It is sometimes difficult to differentiate NASH with mild steatosis from chronic hepatitis of unknown cause using ultrasonography. Tanaka et al.31) reported that BMI and serum ferritin are more useful than abdominal sonography in differentiating between chronic hepatitis and NASH.
Natural history and pathophysiology of NAFLD in children remains unclear. Since the spectrum of NAFLD extends from SS to NASH and cirrhosis, it is important to diagnose NASH early and prevent its progress. According to a study, fibrosis was observed in 71% of pediatric NASH patients on liver biopsy32), and 8% of them showed fibrosis of stage 3 or 433). In our study, liver biopsy was conducted in 15 of the elevated ALT patients. On the histological findings, inflammation in and around the portal vein, lobular inflammatory necrosis, and fibrosis were mild. Only 6 (40%) of them showed fibrosis of stage 1. A limitation of our study is the small number of biopsy cases. A larger prospective study in children with ALT elevation underwent liver biopsy is needed to elucidate the relationship between the histological changes and the severity of NAFLD.
With respect to the effect of lifestyle intervention on NAFLD, a study of 84 children with NAFLD diagnosed through liver biopsy showed significant improvement of their insulin resistance, liver enzymes, and liver echogenicity on ultrasonography with lifestyle advice consisting of diet and increased physical activity16). A clinical trial showed that exercise has modest therapeutic effect in reducing visceral fat and improving glucose intolerance34). These may partially explain the beneficial effect of lifestyle intervention in improving the hypertransaminasemia. Cho et al.35) also reported that combining vitamin E and ursodeoxycholic acid treatment with BMI reduction through exercise and dietary therapy improve the patients' clinical symptoms and biochemical profiles, and that drug treatment alone, without BMI reduction, does not improve NAFLD.
In our study, serum ALT was normalized in around half of 37 patients who could be followed up for a year while being treated with lifestyle intervention. There was no correlation of the normalization of posttreatment serum ALT and BMI, serum ALT at diagnosis and ultrasonographic results. The liver ultrasonography did not demonstrate any remarkable change after a year intervention. It is likely that ultrasonography is not sensitive enough to detect a short-term improvement in steatosis.
In this study, liver biopsy findings were compared according to the status of ALT levels after lifestyle intervention in 12 patients with elevated ALT, which demonstrated that those with more severe histological changes tended to have continuously increasing ALT levels. Although the results were not statistically significant because of the small number of patients, histological changes in the liver before treatment seem to be related to treatment response. The activeness and adequacy of treatment are also believed to have had a considerable effect on the outcome.
In conclusion, patients with obesity and ALT elevation are closed related with male sex, high BMI, and severe fatty change on ultrasonography. NAFLD should be considered as explanation for abnormal ALT elevation, particularly in individuals with identifiable risk factors for steatosis. Lifestyle intervention with exercise and diet in children with NAFLD can lead to a significant improvement of ALT. Lifestyle intervention should represent the first step in the management of children with NAFLD. Further study using a larger number of patients may be necessary on the correlation between changes in liver biopsy results and the normalization of posttreatment serum ALT.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.