Clinical importance of F-waves as a prognostic factor in Guillain-Barré syndrome in children
Article information
Abstract
Purpose
A limited number of studies have examined the link between F-wave abnormalities and clinical presentation in pediatric Guillain-Barré syndrome (GBS). Therefore, this study examined the importance of F-wave abnormalities as a prognostic factor in pediatric GBS patients.
Methods
The records and electrodiagnostic studies (EDS) of 70 GBS patients were retrospectively evaluated, and divided into 2 groups according to the results of EDS. Group A (n=33) presented with F-wave abnormalities, and group B (n=26) exhibited normal findings. We compared laboratory reports, clinical features, response to treatment, and prognosis between the 2 groups.
Results
Motor weakness was the most frequently observed symptom for either group. Clinically, the incidence of fever and upper respiratory symptoms differed between the 2 groups, while the prevalence of abnormal deep tendon reflex (DTR) was significantly higher in group A than B (P<0.05). Patients diagnosed with GBS had received intravenous immunoglobulin treatment: 94% in group A and 58% in group B. Furthermore, significantly greater numbers of patients in group A showed H-reflex abnormalities and poor prognosis compared with group B (P<0.05).
Conclusion
This study demonstrated that F-waves are a clinically important prognostic factor in GBS. F-wave abnormalities were associated with abnormal DTR and poor prognosis in patients. Limited studies have examined the link between F-wave abnormalities and clinical results; therefore, further randomized controlled studies are needed to confirm the clinical characteristics and efficacy of treatments.
Introduction
Guillain-Barré syndrome (GBS) is a postinfectious disorder. The main features of GBS are its rapidly progressive bilateral and relatively symmetric weakness of the limbs. Severe cases have symptoms associated with involvement of the respiratory muscles or muscles innervated by the cranial nerves1).
GBS is a common acute postinfectious neuropathy with an annual incidence rate between 1.1 and 1.8 per 100,0002). The prognosis is relatively good. However ~25% of patients require artificial ventilation and 20% are still unable to walk unaided after 6 months3). Clinical symptoms and laboratory findings play important roles in the diagnosis of GBS, and electrodiagnostic studies (EDS), which consist of electromyography (EMG) and nerve conduction studies, are used to classify different subtypes of the disease4).
An F-wave study is often used to measure nerve conduction velocity and is particularly useful for evaluating conduction problems in the proximal region of nerves5). The name "F-wave" is derived from the initial recordings, performed in the small muscles of the foot6). The F-wave is a compound action potential evoked by supramaximal antidromic stimulation of a motor nerve. The F-wave pathway involves antidromic excitation of all stimulated motor axons traveling to the spinal cord with reactivation of a small proportion of the anterior horn cell axon hillocks and orthodromic action potentials of one or more motor axons traveling to the muscle7). Few studies have examined the correlations between the presence of F-wave abnormalities and clinical course, response to treatment, and prognosis. We postulated that patients with abnormal F-wave findings have distinct clinical features, laboratory findings, and prognoses. This study examined the clinical importance of F-wave abnormalities as a prognostic factor in pediatric GBS patients.
Materials and methods
1. Subjects
We retrospectively evaluated the medical records and EDS results of 70 GBS patients managed at the Departments of Pediatrics, Yeungnam University Hospital and Kyungpook National University Hospital between January 2004 and December 2014.
The patients evaluated presented to the Department of Pediatrics with characteristic clinical features of GBS, i.e., motor weakness, neuropathic pain, numbness, or gait disturbance. Most also had clinical features of infection or inflammation, such as fever, cough, rhinorrhea, vomiting, or diarrhea, before visiting the hospital. The patients were diagnosed as having GBS based on clinical features, cerebrospinal fluid (CSF) studies, identification of autoimmune antibodies, and EDS. Exclusion criteria were deficient medical records or diagnosis of other neuromuscular diseases, such as transverse myelitis, acute demyelination, and encephalomyelitis.
The patients were divided into 2 groups according to the results of EDS, including F-waves: group A consisted of subjects presenting abnormalities and group B consisted of those with normal findings.
We compared laboratory findings, clinical features until diagnosis, response to treatment, and prognosis between the 2 groups. Inability to walk after 6 months, presence of autonomic dysfunction, and use of a mechanical ventilator are poor prognostic factors, and we considered patients with more than one of these symptoms as having a poor prognosis23).
2. Laboratory findings
1) Autoantibodies, etc.
Blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels were examined to evaluate infection and severity of inflammation. We sampled serum from GBS patients to identify autoantibodies, such as anti-GM1 and anti-GD1a antibodies.
2) CSF analysis
Analysis of CSF was performed to exclude other central nervous system diseases, such as encephalitis, meningitis, and meningoencephalitis.
We measured white blood cell counts, glucose levels, and protein levels in the CSF. While some protein is normally present, an elevated level without an increase in the white blood cell count in the CSF may be indicative of GBS.
3. Electrodiagnostic study
1) Nerve conduction velocity
Nerve conduction velocity, i.e., the speed at which impulses travel through nerves, was examined using electrodes placed on the skin over peripheral nerves to measure the amount of time required for an impulse to travel between electrodes8).
The F-wave is a compound action potential evoked by supramaximal antidromic stimulation of a motor nerve. Prolonged F-wave latencies and the absence of an F-wave in nerves with a normal compound muscle action potential amplitude are highly specific for demyelination. Delayed latency or absence of F-waves differ depending on patient age9101112).
2) Electromyography
EMG was performed to measure the electrical activity of muscle fibers. The EMG test measures the electrical activity within muscle fibers by placing a needle electrode through the skin directly into the muscle and measuring the electrical activity of the muscle. It is usually performed in conjunction with nerve conduction velocity tests13).
4. Statistical analysis
Statistical analysis was performed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). When a significant difference was found between groups, the chi-square and Fisher exact tests were used for pairwise comparisons. In all analyses, P<0.05 was taken to indicate statistical significance.
Results
A total of 70 children were diagnosed with GBS and underwent treatment at Kyungpook National University Hospital and Yeungnam University Medical Center between January 2004 and December 2014. These children had symptoms of upper respiratory tract infection (URI) and acute gastroenteritis in addition to neurological symptoms, including motor weakness and neuropathic pain. Laboratory tests and a CSF examination were performed along with serological examinations, including autoantibody tests. There were no differences between the normal and abnormal groups. EDS was performed to diagnose GBS. While EDS was conducted in 59 individuals, it was not conducted in the remaining 11 due to numerous reasons, such as favorable progress.
The patients were divided into 2 groups: group A consisted of 33 subjects presenting with F-wave abnormalities, while group B consisted of 26 subjects with normal findings on F-wave studies. No response, delayed latency, decreased persistence, and low amplitude were considered abnormal F-wave findings. For H-reflex, 14 subjects in group A showed abnormal findings, whereas none in group B showed any abnormalities.
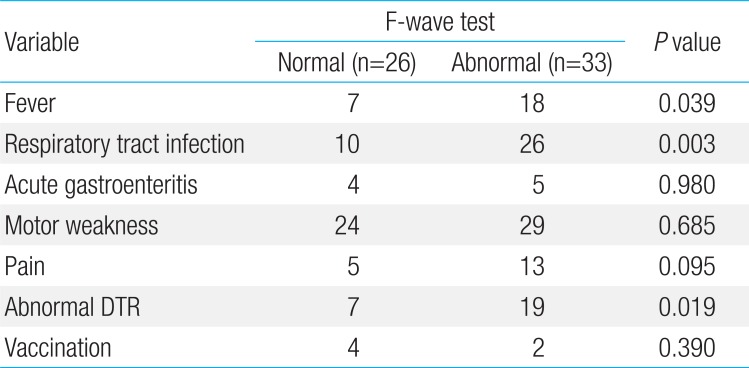
Data regarding age and gender of the children, period between clinical symptom onset and presentation to the hospital, and period between initial visit and examination in each group are presented in Table 1.
The most common clinical symptom in both groups was motor weakness, which was seen in 29 patients (88%) in group A and 24 patients (92%) in group B; the difference between the 2 groups was not significant. The rates of upper respiratory symptoms, including fever, cough, and rhinorrhea, were also high in both groups. In addition, reduced deep tendon reflex (DTR) was also observed. The rates of fever, URI symptoms, and decreased or absent DTR were significantly higher in group A compared with group B (P<0.05).
Table 2 summarizes the clinical differences between groups A and B.
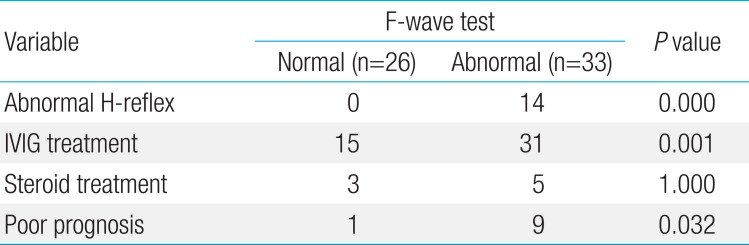
The patients diagnosed based on the clinical symptoms and examination findings outlined above were given intravenous immunoglobulin (IVIG) treatment: 31 of 33 (94%) in group A and 15 of 26 (58%) in group B. Ten patients among those given IVIG treatment in group A who underwent immunotherapy including two or more sessions of IVIG treatment as the first treatment did not show a sufficient treatment effect. Nevertheless, nine of these patients showed unfavorable progress, such as voiding dysfunction, enuresis, and inability to walk independently even 6 months after treatment.
The rates of H-reflex abnormalities and poor prognosis were significantly higher in group A than group B (P<0.05). Differences in the EDS findings, treatment, and prognoses between groups A and B are summarized in Table 3.
DISCUSSION
GBS is a rapidly progressive motor disorder associated with an absence of reflexes. Sensory symptoms in the legs usually mark the onset of the disease, followed by rapidly progressive distal weakness that soon spreads proximally14). In typical cases, the first symptoms are pain, numbness, paresthesia, or weakness in the limbs. Weakness may affect all limb muscles equally, or predominantly the distal or proximal muscles in the arms and legs. Patients show decreased or absent DTR, at least in the affected limbs.
This syndrome is a common postinfectious disorder, and serological studies have shown that Campylobacter jejuni, Epstein-Barr virus, and Cytomegalovirus are the most frequent antecedent infections. Several reports have suggested associations of GBS with Mycoplasma pneumoniae, influenza, and varicella zoster viral infection14). Clinical and laboratory findings play important roles in the diagnosis of GBS2). Typically, the CSF shows increased protein levels with a normal CSF white cell count. A common misunderstanding is that CSF protein levels are always increased in GBS; CSF protein concentrations in patients with GBS are often normal the first week, but increase in more than 90% of patients by the end of the second week1).
Although these findings are important for diagnosis, EDS is the basis of classification of the different disease subtypes. EDS, which usually provides the first evidence of demyelination and is often the only positive evidence among other laboratory tests, is very important for demyelinating polyneuropathy15). In addition, EDS can guide clinicians in predicting the prognosis of patients with GBS4). Early in the course of the syndrome, assessments frequently show small action potentials, prolonged distal motor latency, delayed F-waves, and a conduction block. Occasionally, the results of the first study are normal, and a repeat study is required to document a peripheral nerve disorder14).
F-wave studies are often used to measure nerve conduction velocity and are particularly useful for evaluating conduction problems in the proximal regions of nerves. The F-wave is a compound action potential evoked by supramaximal antidromic stimulation of a motor nerve16).
The absence or slowing of F-waves may be an isolated conduction abnormality, especially during the early stages of illness17). If analyzed correctly, the F-wave is the most sensitive and reliable nerve conduction study for evaluating polyneuropathies, and it can be abnormal in focal proximal nerve dysfunction, can be used to evaluate radiculopathy, and can provide a meaningful physiological window into disorders of the central nervous system18).
The treatment of choice for GBS is IVIG or plasmapheresis. Plasma exchange (PE) is beneficial when applied within the first 4 weeks after onset, but the greatest effect is seen when started early within the first 2 weeks1). Van Doorn et al.3) published the first randomized controlled trials on the use of IVIG in 1992 and showed that IVIG is as effective as PE. Recently, IVIG has replaced PE as the preferred treatment in many centers, mainly because of its greater convenience and availability.
The prognosis of GBS is generally favorable2). However, approximately 25% of patients require artificial ventilation, and 20% are still unable to walk unaided after 6 months3). Despite the demonstrated efficacy of PE and IVIG, GBS remains a disabling disease in a significant proportion of patients, and these treatments have not improved the mortality rate2).
To date, there have been few studies on the relationship between patients' clinical characteristics and F-wave results. A number of studies related to the poor prognostic factors of GBS have been conducted recently. Advanced age, higher disability score at the time of presentation, diarrhea, and mechanical ventilation were reported to be unfavorable prognostic factors3). Furthermore, the prognosis may be predicted by determining the degree of peripheral nerve injury based on the EDS results. This retrospective study was performed to investigate the differences in terms of clinical characteristics, progression, and prognosis between patients with abnormal F-wave results and the normal group, and this had clinical significance as there have been few studies regarding this issue to date.
Fisher18) reported that F-wave studies are most sensitive in detecting acquired demyelinating polyneuropathies, in which they may be quite prolonged, but F-waves are far less sensitive than are EMG studies for assessing motor involvement. F-wave studies were also useful for diagnosing demyelinating polyneuropathies in this study, and the group with abnormal F-wave findings was confirmed to show a significantly poorer prognosis than that of the normal group. However, F-waves and EMG were not compared to verify which one is more useful based on motor involvement. Therefore, further studies are required.
This retrospective study was performed in children who visited tertiary hospitals located in the Daegu and Kyungpook region, were diagnosed with GBS, and underwent treatment between January 2004 and December 2014. This study had a limitation in that the initial examination and treatment were performed at other hospitals before referral to the 2 participating centers. In addition, many patients exhibited clinical symptoms severe enough to require a visit to a tertiary hospital.
Diagnosis of GBS requires consideration of clinical features as well as a CSF exam and EDS. The "albuminocytological dissociation" of CSF can be confirmed by examining the CSF, and demyelination or axonal damage can be confirmed by EDS only when the examination is performed approximately 2 weeks after the onset of clinical features1).
Time points for the CSF and EDS examinations were not standardized. Especially, 11 of 70 children who visited hospitals did not undergo EDS, as they showed favorable clinical progress. Moreover, the CSF examinations and EDS were conducted relatively quickly, usually within 1 week after the onset of clinical features in most cases. In addition, examination of the presence of autoantibodies was not conducted uniformly. The time difference in the EMG/NCV examinations between the normal and abnormal groups was not significant (P=0.789). In the future, we need to control the timing of the examinations prospectively, and serial examinations will be meaningful to confirm statistical significance.
In most cases, GBS is a postinfectious disorder that presents with motor weakness after the onset of symptoms, including fever, URI symptoms, and acute gastroenteritis symptoms. Many cases in the group presenting with abnormal F-waves with preceding fever and URI symptoms exhibited significantly decreased or absent DTR, and this group was more likely to show a poor prognosis compared with the normal group.
Acknowledgments
This research was supported by a grant from the Chunma medical research foundation, Korea, 2015, and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A2060062).
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.