Concomitant use of corticosteroid and antimicrobials for liver abscesses in patients with chronic granulomatous disease
Article information
Abstract
Chronic granulomatous disease (CGD) is a rare inherited disorder caused by defective nicotinamide adenine dinucleotide phosphate oxidase enzyme and characterized by recurrent bacterial and fungal infections. Although liver abscess is a common manifestation of CGD, its management in CGD patients is not well-defined. In addition, the generalized guidelines for treating liver abscesses do not necessarily apply to CGD patients. Corticosteroids are commonly used to control granulomatous complications, such as inflammatory gastrointestinal and genitourinary lesions, in patients with CGD, Corticosteroids have also been used in combination with antimicrobials to treat refractory infections in patients with CGD. Because corticosteroids are capable of suppressing symptomatic inflammation, all potential infections must be adequately controlled prior to corticosteroid initiation. We report 3 typical CGD cases with liver abscesses refractory to conventional treatments that were successfully treated with the concomitant use of corticosteroid and antimicrobials. It remains unclear whether corticosteroid therapy is required for liver abscesses in CGD refractory to conventional treatments. However, based on our observations, use of corticosteroids in combination with optimal antimicrobials should be considered for refractory liver abscesses in CGD.
Introduction
Chronic granulomatous disease (CGD) is a rare primary immunodeficiency caused by mutations in any one of 5 genes encoding subunits of the phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. The functional activity of NADPH oxidase is significantly diminished or completely absent in patients with CGD, resulting in very low or no production of superoxide derivatives, which are important for killing invading microorganisms12).
Infections in patients with CGD are typically caused by a narrow spectrum of bacteria and fungi, such as Staphylococcus aureus, Klebsiella pneumoniae, Serratia marcescens, Burkholderia cepacia, Aspergillus species, and Candida species1234). Recurrent infections occur primarily in the lung, skin, lymph nodes, and liver. Although liver abscesses are observed in about 30%-35% of CGD patients45, the management of liver abscesses in CGD patients is not well-defined. At this time, the generalized guidelines for treating liver abscesses do not necessarily apply to CGD patients. Recently, some studies have reported that concurrent therapy with corticosteroids and antimicrobials is highly effective in CGD patients with liver abscesses567.
We report three typical CGD cases with liver abscesses refractory to conventional treatments that were successfully treated with the concomitant use of corticosteroid and antimicrobials.
Case reports
1. Case 1
A 23-year-old female with CGD was referred for a persistent fever. She was diagnosed with p22phox-deficient (phox, phagocytic oxidase) CGD at 7 years of age. She had multiple pneumonias, lymphadenitis, cellulitis, osteomyelitis, and liver abscess. She had been undergoing prophylactic therapy with interferon-gamma (IFN-γ). Her younger sister was also diagnosed with CGD at 5 years of age (Fig. 1A). One month prior to the current presentation, she was admitted to another hospital with fever, malaise, and chest pain during breathing. Abdominal ultrasound and computed tomography showed a 2 cm-sized abscess in the right hepatic lobe. Treatment with vancomycin, imipenem, and metronidazole reduced the fever after 10 days, but the fever recurred after 3 days. Although amphotericin B was administered, fever continued intermittently for a further 2 weeks.
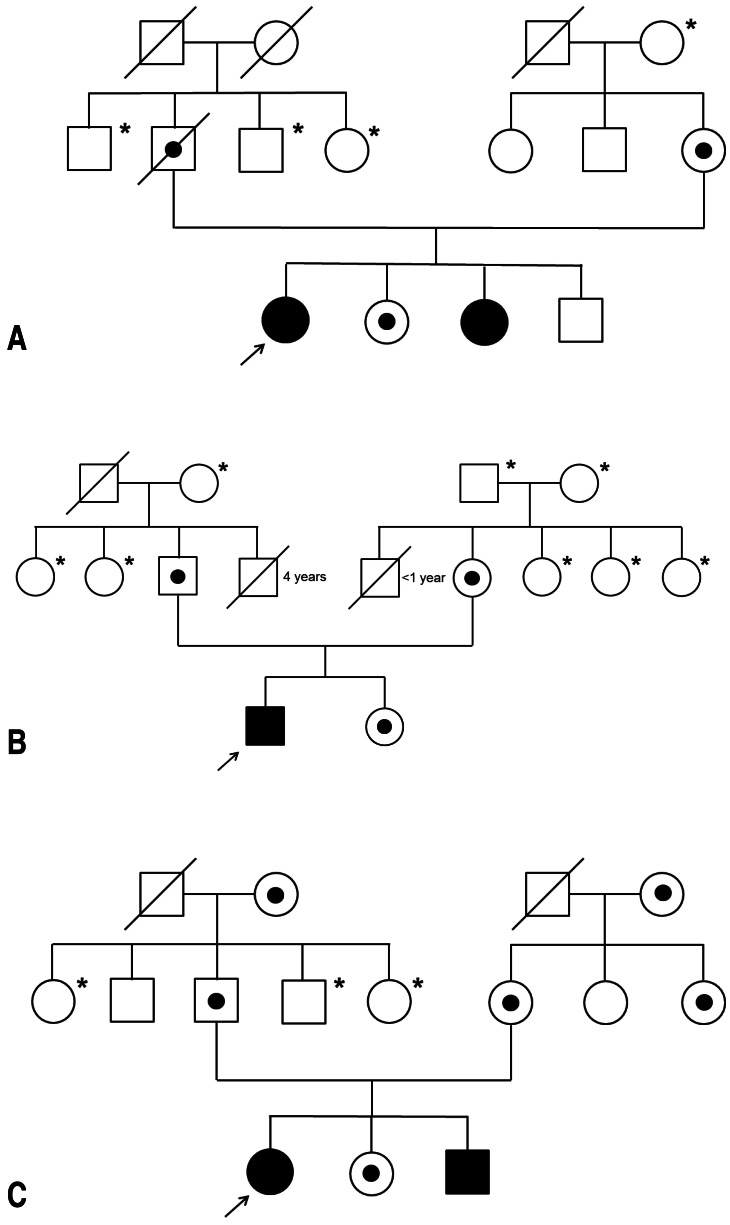
Case pedigrees: (A) pedigree of case 1, (B) pedigree of case 2, and (C) pedigree of case 3. *Not analyzed.
On transfer to Jeju National University Hospital, she had abdominal pain and fever. Laboratory data showed elevated white blood cell (WBC) count (15,300/mm3), liver enzyme alanine aminotransferase 96 IU/L, and aspartate aminotransferase 113 IU/L, and inflammatory markers erythrocyte sedimentation rate (ESR) >120 mm/hr and C-reactive protein (CRP) 232.7 mg/L. Computed tomography of the abdomen showed multiple abscesses in the right lobe of the liver (Fig 2A, B). Two large abscesses in the dome (5 cm×4.5 cm) and inferior portion (4 cm×2.5 cm) of the liver were aspirated and percutaneously drained. Antibiotic treatment was initiated with vancomycin, clindamycin and meropenem. She remained febrile for the first 2 weeks of hospitalization, but cultures of the blood and aspirate were negative. Due to persistence of the fever and abscess despite antibiotic treatment, the drains were removed and intravenous methylprednisolone (1.0 mg/kg/day) was administered. She showed rapid clinical improvement within 1 week with significant decreases in the size of the abscesses, and inflammatory markers also decreased (ESR, 71 mm/hr; CRP, 22.5 mg/L). After weaning of intravenous methylprednisolone over 4 weeks to 0.25 mg/kg/day, followed by transition to trimethoprim/sulfamethoxazole (TMP/SMX), cefdinir, and oral prednisone (0.25 mg/kg/day), she was discharged. Abdominal ultrasound 1 month after discharge showed only a residual 3.5 cm lesion in the liver dome (Fig. 2C). Oral prednisone was tapered and stopped over 3 months.
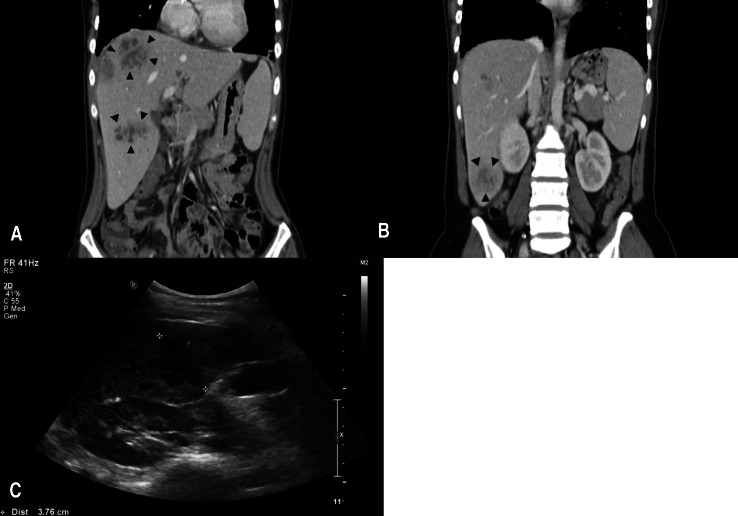
Computed tomography (CT) and abdominal ultrasound for case 1. The coronal view on enhanced CT shows multiple abscesses in the right lobe of the liver, with 2 large abscesses in the dome (5 cm×4.5 cm, black arrowheads, (A) and inferior portion (4 cm×2.5 cm, black arrowheads, (B) of the liver. (C) The abdominal ultrasound image obtained 5 months after initial presentation shows a reduction in size (3.5 cm) of the liver dome.
2. Case 2
A 21-year-old male was admitted after 1 week of fever while on prophylactic TMP/SMX and IFN-γ therapy. He was diagnosed with p22phox-deficient CGD at 14 years old, based on multifocal liver abscesses. While he had experienced pneumonias, lymphadenitis, cellulitis, and osteomyelitis, he was not diagnosed with CGD until this time. Abdominal ultrasound on admission showed a 4×2 cm-sized abscess in the left hepatic lobe. Laboratory data showed an elevated WBC count (20,500/mm3) and inflammatory markers (ESR, 75 mm/hr; CRP, 114.6 mg/L). Antibiotic treatment was initiated with vancomycin, cefotaxime, and metronidazole. He remained febrile for a further 2 weeks and blood cultures were negative. The abscess was aspirated using ultrasound guidance. The antibiotic regimen was changed to vancomycin, clindamycin and meropenem, and intravenous methylprednisolone (0.5 mg/kg/day) was initiated. The fever gradually subsided during the following 2 weeks and follow-up imaging of the abdomen showed significant decreases in the size of the abscesses. Intravenous methylprednisolone was tapered over 2 weeks to 0.15 mg/kg/day, followed by transition to oral prednisone (0.1 mg/kg/day). Over the next 3 months, liver lesions completely resolved despite the discontinuation of oral prednisone and antibiotics.
3. Case 3
A 14+6-year-old female with p22phox-deficient CGD was admitted with 4 days of fever, cough, nausea, chills and myalgias. She was diagnosed with CGD at 12 months of age and had experienced pneumonia, lymphadenitis, cellulitis, and osteomyelitis on several occasions. Her younger brother was also diagnosed with CGD shortly after the birth (Fig. 1C). Prophylactic TMP/SMX and IFN-γ therapy had been voluntarily discontinued for more than 1 year before admission. Chest radiographs on admission showed pneumonic consolidation of the right upper lung field (Fig. 3A), and inflammatory markers (ESR, 116 mm/hr; CRP, 159.7 mg/L) were elevated. She was initially treated with ampicillin/sulbactam and cefotaxime for pneumonia. Fever continued intermittently for 2 weeks. Ampicillin/sulbactam was stopped and vancomycin and amphotericin B were added to the treatment. However, after the new regimen was initiated, she was febrile for a further 1 week. Surveillance abdominal ultrasound identified three large lobulated cystic lesions in the right liver dome (7.7 cm) and left lateral (5.0 cm) and medial segment (2.8 cm) of the liver (Fig. 3B, C). Inflammatory markers (ESR>120 mm/hr; CRP, 220.3 mg/L) were elevated. Two large abscesses in the dome and lateral segment of the liver were percutaneously drained, and intravenous methylprednisolone (1.0 mg/kg/day) was started. She gradually improved during the following 10 days. After 2 weeks of intravenous methylprednisolone, methylprednisolone was weaned over 2 weeks. However, at a dose of 0.25 mg/kg of methylprednisolone, fever recurred with increased inflammatory markers. Intravenous methylprednisolone (0.75 mg/kg/day) was restarted and more slowly weaned over 3 weeks to 0.25 mg/kg/day. Intravenous methylprednisolone were transitioned to oral prednisone (0.25 mg/kg/day) and she was discharged. Oral prednisone was tapered and stopped with the antibiotics over 3 months. Subsequent abdominal ultrasound performed 13 months after initial presentation showed complete resolution (Fig. 3D).
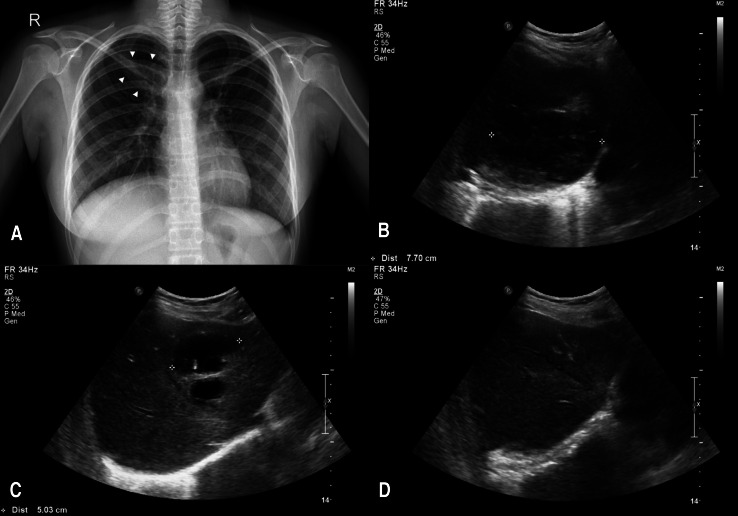
Chest radiography and abdominal ultrasound for case 3. (A) Chest radiograph on admission shows a pneumonic consolidation (white arrowheads) in the right upper lung field black. (B, C) Abdominal ultrasound shows 3 large lobulated cystic lesions in the right liver dome and left lateral and medial segments of liver. (D) Abdominal ultrasound obtained 13 months after initial presentation shows complete resolution.
Discussion
CGD is an inherited primary immunodeficiency resulting from defects in any one of the subunits of the phagocyte NADPH oxidase12). The NADPH oxidase complex is composed of 5 major subunits. Two of these, gp91phox and p22phox, are membrane-bound components encoded by the CYBB and CYBA genes, respectively. The remaining 3 components include p47phox, p67phox, and p40phox, encoded by the corresponding genes, NCF1, NCF2, and NCF412). The X-linked form of CGD is caused by mutations in CYBB, and accounts for approximately 70% of cases. The autosomal recessive forms are caused by mutations in CYBA, NCF1, and NCF2, accounting for approximately 5%, 20%, and 5% of cases, respectively89). Only one case with a mutation in NCF4 has been reported to date10). The incidence of CGD is 3-4 per 1,000,000 individuals1234), and the prevalence of CGD in Korea is similar to that in other regions11). On the other hand, the prevalence of CGD on Jeju Island is 20.7 per 1,000,000 individuals, which is 10×50 folds higher than in other regions of Korea12). We hypothesized that the high prevalence of CGD on Jeju Island was associated with the same mutation inherited from a common proband, and we demonstrated that all the CGD patients on Jeju Island had an identical and homozygous mutation in the CYBA gene; c.7C>T in CYBA exon 1, and p.Q3X in p22phox.12) All patients with CGD on Jeju Island were presumed to be the A220 phenotype (where A indicates autosomal recessive, and the superscript 0 indicates a complete absence of the affected subunit)12).
The majority of CGD patients with liver abscesses have prior histories, such as cases 1 and 2. With or without surgery, liver abscesses are relatively easily controlled, but commonly recur with a different organism713). It is known from previous reports that a prior liver abscess is an important risk factor for recurrence57). This may be because hepatic dysfunction with portal venopathy and nodular regenerative hyperplasia is strongly associated with liver abscesses in CGD patients14). It is thought that liver regeneration or microvascular insults after repeated abscesses cause nodular regenerative hyperplasia and portal venopathy, and the altered hepatic architecture or blood flow results in subsequent infection713). However, whether there is a cause-and-effect relationship between repeated abscesses and altered hepatic architecture or blood flow remains unclear.
Although liver abscess is a common manifestation of CGD, few reports describe the management of liver abscesses in patients with CGD. In the past, hepatic resection, evacuation of the exudate, external drainage, and long-term continuous antibiotic therapy has been recommended5). The majority of cases used percutaneous aspiration of hepatic abscesses for isolation of the organism before use of antibiotics and surgical management. Since liver abscesses in patients with CGD are septated masses with a fibrous pseudo-capsule and thick condense fluid, most attempts at aspiration and drainage of hepatic abscesses are unsuccessful5). In our cases, liver abscesses were aspirated and drains were placed percutaneously, but the amount of aspirate was minimal and drainage was unsuccessful. Rather, the percutaneous drain seemed to be a focus of the secondary liver infection. Lublin et al.5) recommended the administration of ceftriaxone and vancomycin with or without rifampin because S. aureus was the most common isolate from liver abscesses.
antimicrobial therapy, but it is difficult to identify the causative pathogen in the majority of clinical CGD cases. In our case, cultures from blood and aspirates of liver abscess were all negative. While treating liver abscesses refractory to proper antimicrobial coverage against potential pathogens, we used corticosteroids to improve symptoms and ameliorate aberrant inflammation. It has been shown that liver abscesses in patients with CGD can be treated with systemic corticosteroids in addition to antimicrobials67).
Corticosteroids are commonly used to control granulomatous complications in patients with CGD, such as inflammatory gastrointestinal and genitourinary lesions3). Corticosteroids have also been used in combination with antimicrobials to treat refractory infections in patients with CGD367). Patients with CGD display signs of dysregulated inflammation, which is not only associated with deficient superoxide and related reactive oxygen intermediates. Although several mechanisms of chronic and hyperinflammation in CGD had been proposed, it cannot be fully explained at present. However, the hyperinflammation in CGD may be associated with the intracellular persistence of microorganisms and could inhibit the delivery of antimicrobials to the cells6). Corticosteroids decrease the activation, proliferation, and differentiation of many cells involved in inflammation67). In the case of refractory infections in patients with CGD, concomitant administration of corticosteroids with antimicrobials decreases the severe inflammatory response and thus seems to effectively manage the refractory infections.
Case 1 in this report showed multiple abscesses and progressive liver abscesses despite optimal antimicrobial coverage. Corticosteroids were started at a dose of 1 mg/kg daily and continued for 2 weeks before tapering off over several months. Although there was no uniform dose of corticosteroid, low or moderate anti-inflammatory doses (about 1 mg/kg per day of prednisone) are recommended67). Because corticosteroids are capable of suppressing symptomatic inflammation, all potential infections must be adequately covered prior to corticosteroid initiation. Use of corticosteroid without optimal antimicrobial coverage can have negative consequences in CGD615).
As seen in case 3, rapid tapering of corticosteroid may be associated with exacerbation of inflammation and liver abscesses. Because long-term use of corticosteroids may cause opportunistic infections and have metabolic effects, the appropriate duration of tapering from our experience was not more than 3 months. The necessity of intravenous antimicrobials is also unclear, but the concomitant use of corticosteroid with antimicrobials is reasonable and safe for unexpected infections.
It remains unclear whether the corticosteroid therapy is helpful or required for liver abscesses refractory to conventional treatments. Moreover, there was no uniform regimen for corticosteroids therapy, such as dosage, duration, duration of tapering, the necessity for coadministration of antimicrobials, and the comparative advantages over surgical treatment. Controlled prospective studies are needed to determine the safety and proper regimen for corticosteroid therapy in CGD.
In conclusion, we report three typical cases of CGD with liver abscesses refractory to conventional treatments that were successfully treated with the concomitant use of corticosteroids and antimicrobials. Based on our observations, concomitant use of corticosteroids in combination with optimal antimicrobials should be considered for refractory liver abscesses in CGD.
Acknowledgment
This research was supported by the 2014 scientific promotion program funded by Jeju National University.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.