Correlation of B-type natriuretic peptide levels and echocardiographic parameters in preterm infants with patent ductus arteriosus
Article information
Abstract
Purpose
This study aimed to evaluate the correlation, according to postnatal age, between plasma B-type natriuretic peptide (BNP) levels and echocardiographic parameters for the assessment of patent ductus arteriosus (PDA) in preterm infants with respiratory distress.
Methods
We enrolled 42 preterm infants with respiratory distress who underwent serial echocardiographic evaluation with simultaneous plasma BNP measurements until ductal closure. The correlations between BNP levels and the following 4 representative echocardiographic parameters were studied: diameter of the ductus arteriosus (DA), ratio of the left atrial diameter to the aortic diameter (LA/Ao), ratio of the PDA diameter to the infant's left pulmonary artery diameter (PDA/LPA), and the antegrade diastolic flow of LPA (DFLPA).
Results
BNP levels were significantly correlated to the magnitude of the ductal shunt, comprising the DA diameter, PDA/LPA ratio, LA/Ao ratio, and antegrade DFLPA for the overall study period. The earliest significant correlation, starting from postnatal day 2, was observed between the LA/Ao ratio and BNP levels. The PDA/LPA ratio and the antegrade DFLPA showed significant correlations with BNP levels postnatal day 3 onward, and with the DA diameter, postnatal day 5 onward.
Conclusion
BNP levels and echocardiographic parameters showed a positive correlation, but the significance of the correlations differed according to the postnatal age, especially during the first few days of life.
Introduction
A hemodynamically significant patent ductus arteriosus (hsPDA) is a common cardiovascular disorder in preterm infants, especially in those with respiratory distress. Morbidities such as intraventricular hemorrhage, necrotizing enterocolitis, bronchopulmonary dysplasia, and mortality associated with hsPDA are serious problems123456). Therefore, accurate diagnosis and optimal management of hsPDA are important for improving the clinical outcomes of preterm infants with a PDA.
Over the past decade, plasma B-type natriuretic peptide (BNP) levels have been proposed as a tool for the prediction and diagnosis of hsPDA in preterm infants, especially given the commercial availability of a bedside testing kit78910111213141516). A recent systematic review evaluating the diagnostic accuracy of BNP for hsPDA suggests that measurement of BNP levels seems to be an accurate test for identifying hsPDA in preterm infants, with an estimated summary sensitivity and specificity of 0.88 and 0.92, respectively17).
In most studies evaluating the role of BNP as a diagnostic tool for hsPDA, positive correlations between BNP levels and echocardiographic parameters indicating the magnitude of the ductal shunt have been reported15161718). Several other studies1216) have also shown that these correlations varied according to postnatal age, and were especially low during the first few days after birth in preterm infants. However, a comprehensive study about serial and simultaneous comparisons of daily BNP levels and various echocardiographic parameters during each of the first few days of postnatal life and whether this early relationship shows similar patterns in later postnatal days, has not yet been performed.
The aim of this study was to evaluate the correlation of plasma BNP levels with echocardiographic parameters in assessing PDAs in preterm infants with respiratory distress according to postnatal age.
Materials and methods
1. Study population and design
This study was performed retrospectively by reviewing the medical records of Korean infants admitted to the neonatal intensive care unit at the Korea University Ansan Hospital between August 2013 and August 2015. The inclusion criteria for this study comprised infants: (1) born at a gestational age of 25 to 36 weeks, estimated from the last menstrual period; (2) admitted within 24 hours after birth; (3) no major congenital malformation, including cardiac abnormalities except for patent foramen ovale; (4) postnatal respiratory distress requiring ventilation assistance; and (4) an echocardiographic study for PDA started within 72 hours after birth. This retrospective observational study protocol was approved by the Institutional Review Board of Korea University Ansan Hospital (AS15148) with a waiver for parental consent.
During the study period, 42 preterm infants with respiratory distress who had a ductal patency were enrolled. Serial echocardiographic evaluations with simultaneous plasma BNP measurements were performed up to the 14th postnatal day. Patients were analyzed on a day-to-day basis up to the 4th postnatal day, and matching cases were grouped respectively into postnatal days 5 and 6, 7 to 9, and 10 to 14 for the acquisition of a sufficient number of cases.
2. Echocardiographic studies
A Vivid q cardiovascular ultrasound scanner (GE Healthcare, Haifa, Israel) with a 3.5- to 8.0-MHz pediatric cardiac transducer was used, incorporating 2D, harmonics, M-mode, and color, pulsed, and continuous wave Doppler. To estimate the magnitude of left-to-right shunting across the PDA, 4 representative echocardiographic parameters including the diameter of the ductus arteriosus (DA), the ratio of the left atrial diameter to the aortic diameter (LA/Ao), the ratio of the diameter of the PDA to the infant's left pulmonary artery diameter (PDA/LPA), and the antegrade diastolic flow of the left pulmonary artery (DFLPA) were evaluated1920). All studies were performed by a single echocardiographer to avoid any interobserver variability. Three to five consecutive measurements were taken on each view, and the average was used as the final value to reduce intraobserver variability.
3. Measurement of plasma BNP levels
When ductal patency was confirmed by echocardiography, at the time of performing the Doppler echocardiographic measurements, blood samples for the measurement of BNP levels were collected via peripheral venous puncture or in rare cases, umbilical artery catheter aspiration. BNP levels were detected with a commercial kit (Alere Triage Meterpro, San Diego, CA, USA) by using a fluorescence immunoassay made available for bedside use, which requires 250 µL of whole blood. The measurable range was from 5 to 5,000 pg/mL.
4. Statistical analyses
Data were analyzed using the IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). Categorical data are presented as numbers (%) and continuous data as mean±standard deviation or median (25th–75th percentile). The correlation between BNP levels and echocardiographic parameters was analyzed using bivariate analysis with Spearman correlation. A P value of <0.05 was considered significant.
Results
1. Patient characteristics
Forty-two eligible infants were enrolled during a 2-year period (from August 2013 to August 2015). Their mean gestational age was 30 weeks (range, 25–36 weeks), and the mean birth weight was 1,503 g (range, 550–3,130 g). Nineteen (45.2%) were male infants, and 29 (69.0%) had received some antenatal steroids. The mean Apgar score was 6 and 8 at 1 and 5 minutes postbirth, respectively. Of the 42 preterm infants, 28 infants (66.7%) received exogenous surfactant administration for respiratory distress. All eligible infants required some kind of respiratory support and 30 (72.4%) required invasive ventilation with endotracheal intubation. Twenty-three infants (54.8%) required medical therapy, and 9 (21.4%) needed surgical ligation for closure of the PDAs (Table 1).
2. Correlation between the BNP levels and the diameter of the DA
There was a significant correlation between BNP levels and the diameter of the DA (r=0.368, P<0.001) for the overall study period (Fig. 1).
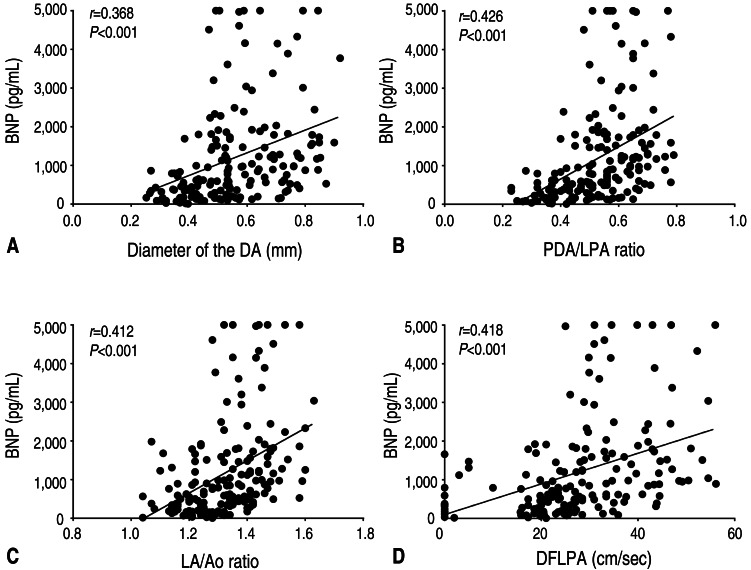
Correlations between B-type natriuretic peptide (BNP) levels and echocardiographic parameters. (A) Correlation between BNP levels and the diameter of the ductus arteriosus (DA; r=0.368, P<0.001). (B) Correlation between BNP levels and the ratio of the patent ductus arteriosus (PDA) diameter to the infant's left pulmonary artery diameter (PDA/LPA ratio; r=0.426, P<0.001). (C) Correlation between BNP levels and the ratio of the left atrial diameter to the aortic diameter (LA/Ao ratio; r=0.412, P<0.001). (D) Correlation between BNP levels and the antegrade diastolic flow of the left pulmonary artery (DFLPA; r=0.418, P<0.001).
When analyzed on a day-to-day basis, there was no significant correlation between the BNP levels and the diameter of the DA until the 4th postnatal day. However, from the 5th postnatal day, the correlations began to show significant correlations (r=0.478, P=0.018). Moreover, BNP levels and the diameter of the DA continued to show significantly positive correlations as the postnatal age increased. The Spearman correlation coefficient ranged from 0.394 to 0.480, and the P value ranged from 0.004 to 0.070 (Fig. 2).
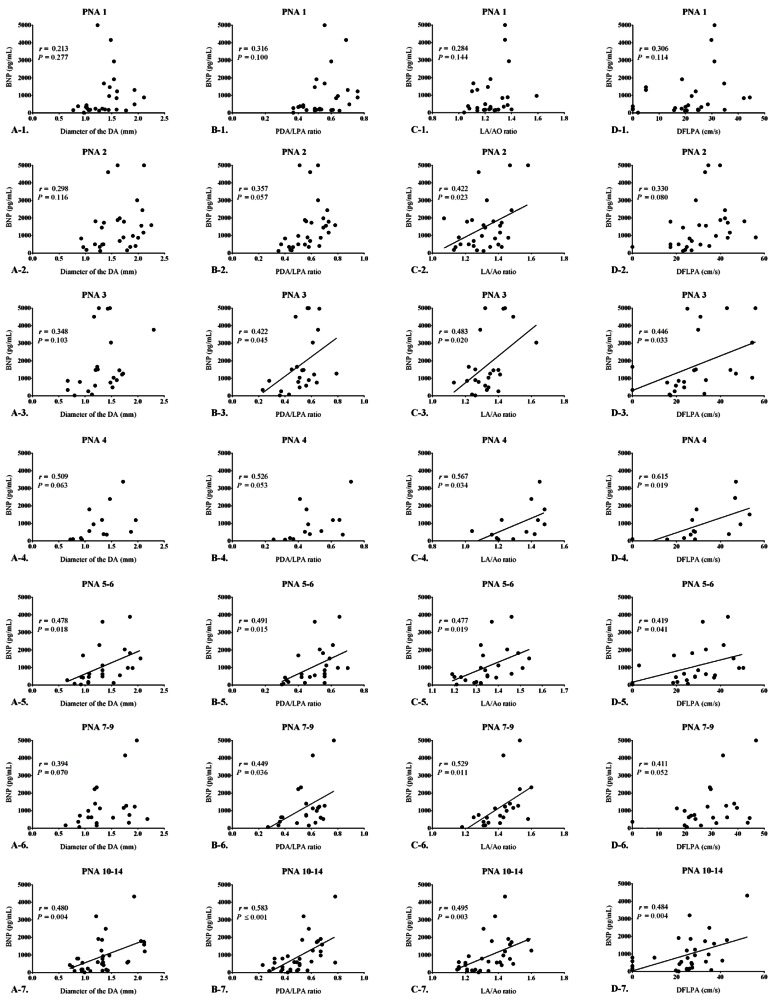
Correlations between B-type natriuretic peptide (BNP) levels and echocardiographic parameters according to age. (A) Correlation between BNP levels and the ductus arteriosus diameter according to age; a significant correlation was observed postnatal day 5 onward (r=0.478, P=0.018). (B) Correlation between BNP levels and the PDA/LPA ratio according to age; a significant correlation was observed postnatal day 3 onward (r=0.422, P=0.045). (C) Correlation between BNP levels and the LA/Ao ratio according to age; a significant correlation was observed postnatal day 2 onward (r=0.422, P=0.023). (D) Correlation between BNP levels and the DFLPA according to age; a significant correlation was observed postnatal day 3 onward (r=0.446, P=0.033). PDA/LPA ratio, the ratio of the patent ductus arteriosus diameter to the infant's left pulmonary artery diameter; LA/Ao ratio, the ratio of the left atrial diameter to the aortic diameter; DFLPA, diastolic flow of the left pulmonary artery; PNA, postnatal age.
3. Correlation between the BNP levels and the PDA/LPA ratio
There was a significant correlation between BNP levels and the PDA/LPA ratio (r=0.426, P<0.001) for the overall study period (Fig. 1).
The PDA/LPA ratio showed no significant correlations up to the 2nd postnatal day. However, BNP levels started to show a significant positive correlation with the PDA/LPA ratio from the 3rd postnatal day (r=0.422, P=0.045). On strict statistical analysis, all the periods did not show significant correlations between the PDA/LPA ratio and BNP levels (PNA 4, 4th postnatal day; r=0.526, P=0.053), but they continued to show a positive trend of correlation as post-natal age increased, with the Spearman correlation coefficient ranging from 0.422 to 0.586, and the P value ranging from <0.001 to 0.053 (Fig. 2).
4. Correlation between the BNP levels and the LA/Ao ratio
There was a significant correlation between BNP levels and LA/Ao ratio (r=0.412, P<0.001) for the overall study period (Fig. 1).
The LA/Ao ratio also showed no significant correlation with BNP levels on the 1st postnatal day, but showed a significant correlation from the 2nd postnatal day onwards (r=0.422, P=0.022). The correlation remained strong and significant for every study period thereafter, with the Spearman correlation coefficient ranging from 0.422 to 0.567, and P value ranging from 0.003 to 0.034 (Fig. 2).
5. Correlation between the BNP levels and the DFLPA
There was significant correlations between the BNP levels and the DFLPA (r=0.418, P<0.001) for the overall study period (Fig. 1).
The DFLPA showed no statistically significant correlations with BNP levels until the 2nd postnatal day, but showed significance from the 3rd post-natal day (r=0.446, P=0.033). Although statistically, not all of the periods, such as the 7th–9th (r=0.411, P=0.052) postnatal day, showed significance, but even during this period, the P value was nearly statistically significant. The DFLPA showed significance with Spearman correlation ranging from 0.411 to 0.615, and P values ranging from 0.004 to 0.052 (Fig. 2).
Discussion
BNP, a cardiac natriuretic hormone, is synthesized and released into the circulation by ventricular cardiac myocytes in respons e to pressure overload, volume expansion, and increase in myocardial wall stress182122). Given the pathophysiological basis of hsPDA, BNP, with its physiologic role, is a likely candidate to be used as a biomarker in the identification of hsPDA. As hsPDA causes left-atrial and, subsequently, left-ventricular overload, leading to the increased production of BNP, BNP along with echocardiographic parameters may have a valuable role in facilitating the early and accurate diagnosis of hsPDA in preterm infants. In our study, BNP levels and echocardiographic parameters showed a strong positive correlation, which reflected the magnitude of the ductal shunt, and showed reliable results during the entire observational period in preterm infants with a PDA.
However, in our previous study16), there was a poor correlation between BNP levels and the magnitude of the ductal shunt using several echocardiographic parameters such as the diameter of the DA, the LA/AO ratio, and the diastolic flow velocity of the left pulmonary artery by echocardiography at 12 and 24 hours of age. In addition, in the study by Flynn et al.12), there were poor correlations between BNP levels and several echocardiographic parameters reflecting the magnitude of the ductal shunt, such as the LA/Ao ratio, the diameter of the DA, and the PDA/LPA ratio at 2 days of age or less when compared with values beyond 2 days of age. Another study by Chen et al.23) has pointed out that although BNP levels and the ductal shunt magnitude are significantly correlated, the results of BNP measurements were variable, rendering BNP levels alone to be insufficient in monitoring hemodynamic changes of PDAs.
In this study, we found that echocardiographic parameters showed a discrepancy with BNP levels for some periods immediately after birth, reflecting the cardiac volume overload due to hsPDA. We evaluated whether a specific postnatal age exists beyond which point BNP levels and echocardiographic parameters could be used to accurately diagnose hsPDA in preterm infants.
In premature infants, the DA is more likely to remain patent, especially in high-risk preterm infants, and shunt flow through the ductus may have important hemodynamic consequences21). Previous studies have shown that an infant with a DA diameter of more than 1.5 mm has a higher likelihood of developing symptomatic PDA, and this value seems to be the point where end-organ hypoperfusion occurs242526).
In our study, the diameter of the DA of the PDA proved to have statistical significance from the 5th postnatal day onwards. This was in parallel to the findings from numerous studies about the relationship between the diameter of the DA and BNP levels. Many studies1214162227) report a significant positive correlation, but results show a difference according to postnatal age. Flynn et al.12) reported that infants aged 2 days and older showed significant positive correlations in neonates, but failed to find any correlation in those less than 2 days old. Lee et al.16) reported that although significant correlations at 12 and 24 hours of birth could not be demonstrated, the trend showed a positive tendency as the postnatal time increased.
The size of the DA can also be estimated as equal to the diameter of the relatively fixed left pulmonary artery28). Ramos et al.29) attempted to identify echocardiographic parameters in infants aged less than 4 postnatal days, in order to predict the subsequent need for closure of a clinically significant PDA in extremely low birth weight infants. Their multiple logistic regression modeling, using several echocardiographic parameters of PDA, showed significance only for PDA size estimated by the PDA/LPA ratio in the first 4 postnatal days.
Our results showed that significance between increased BNP levels and the magnitude of the ductal shunt could only be determined from the 3rd postnatal day onwards. Our results were similar to those of Flynn et al.12), who found that until the 2nd postnatal age, the PDA/LPA ratio did not show any significant correlation with BNP (r2=0.521, P=0.082), but after the 3rd day, the two proved to have significant positive correlations (r2=0.635, P<0.001). Hsu et al.14) who found a significant correlation in infants aged 1.5 days (r2=0.450, P=0.001) did not consider the potential differences incurred by each passing postnatal day. However, the trends of the relationship from both reports are similar, and the PDA/LPA ratio and BNP levels proved to have significance.
The LA/Ao ratio is measured by using echocardiography to estimate the increase in effective pulmonary blood flow. The LA/Ao ratio compares the diameter of the left atrium to the root of the aorta, because the left atrium enlarges as the volume load increases on the left side of the heart due to left to right ductal shunting, while the diameter of the aorta remains relatively stable as it is less affected. The LA/Ao ratio is a semiquantitative method used to measure the amount of blood flowing through the PDA, and shows a significant correlation with increased pulmonary flow that is due to increased ductal flow30).
In our study, the LA/Ao ratio proved to be significant at the earliest time point, compared with the other 2 parameters. Our results showed that already from the 2nd postnatal day, the BNP level had increased significantly in comparison to the LA/Ao ratio, which also increased. This was consistent with the results of previous studies regarding the LA/Ao ratio. Lee et al.10) showed that BNP levels had significant correlations with the LA/Ao ratio at 24- and 48-hour after birth, and was stronger at 48 hours. Choi et al.11) found that on the 3rd postnatal day, the LA/Ao ratio showed a significant positive correlation with BNP levels in preterm infants (r2=0.726, P=0.001). This difference in significance according to postnatal age was again seen in a study by Flynn et al.12); the correlation was significant only in neonates who were more than 3 postnatal days old (r2=0.391, P=0.002).
Normally present in the first few days of life in healthy preterm infants, the anterograde flow velocity produced during diastole in the pulmonary arteries can be explained by the atrium contracting during systole and the subsequent passive filling of the right ventricle313233). The magnitude of the ductal shunt causes pressure differences in the aorta and pulmonary artery, and this pressure difference along with the diameter of the DA, affects the diastolic flow velocity of the LPA. Greater magnitudes of ductal shunts causes increased diastolic flow velocity, and this parameter may be useful as a diagnostic marker of hsPDAs.
Suzumura et al.34) demonstrated that the DFLPA increased as PDAs became symptomatic and could be relied on to indirectly represent the change in ductal shunt volume in preterm infants from 13 hours to 3.5 days after birth (84 hours), with a sensitivity and specificity of 0.82 and 0.83, respectively. Our study compared DFLPA with BNP levels on a daily basis, and found significant positive correlations from the 3rd postnatal day (r=0.446, P=0.033). The results of our study was in parallel to the results of Choi et al.11), who showed that the DFLPA shows a positive correlation with BNP levels (r=0.877, P<0.001) on the 3rd day after birth.
In the present study, the Spearman correlation coefficient was relatively low and significance was poor between BNP levels and the magnitude of the ductal shunt compared with those of previously reported studies9101112151618) evaluating the role of BNP as a diagnostic tool for hsPDA, because our study included infants who did not present with symptomatic PDAs and also those who did not need any treatment. Only 23 infants (54.8%) required medical therapy for symptomatic PDA.
In summary, a significant correlation of BNP levels with echocardiographic parameters was evident from the 2nd postnatal day in the case of the LA/Ao ratio, from the 3rd postnatal day for PDA/LPA ratio and DFLPA, and from the 5th postnatal day for the diameter of the DA. On the 2nd postnatal day, the LA/Ao ratio may be the only echocardiographic parameter useful in diagnosing hsPDA, along with the BNP levels, and the PDA/LPA ratio and the DFLPA or the diameter of the DA should also be taken into consideration from the 3rd or 5th postnatal day, respectively.
The relative lack of correlation during the first few days of life may be attributable to the fact that although pulmonary vascular resistance starts to decrease immediately after birth, the later more gradual decrease results in high pulmonary pressure persisting for several hours to days. Although the diameter of the DA may be the largest immediately after birth, the high pulmonary vascular resistance prevents ductal shunting through the DA and thus have relatively lesser influences on BNP levels.
The limitation of this study is that other factors which may affect BNP levels, besides the hemodynamic effect of PDA, were not considered. Perinatal clinical factors, such as gestational age, birth weight, and the present of asphyxia, lung disease or infection may affect BNP levels, and the different management protocols of neonatal intensive care units such as postnatal fluid management and ventilator care can influence BNP levels. Comorbidities such as pulmonary hemorrhage or hemorrhagic edema, cerebral hemorrhage and whether these were due to the PDAs or not were also not considered. These factors may have significant influences on BNP levels, and further studies defining their impact are needed.
In conclusion, BNP levels and echocardiographic parameters showed a positive correlation, but its significance should be considered differently according to the post-natal age, especially during the first few days of life.
Acknowledgments
We thank the physicians and nursing staff working in the neonatal intensive care unit of Korea University Ansan Hospital for their enthusiastic support and cooperation.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.

