A prospective study to assess the efficacy and safety of oral propranolol as first-line treatment for infantile superficial hemangioma
Article information
Abstract
Purpose
To determine the efficacy and safety of oral propranolol as a first-line treatment for superficially located infantile hemangioma (IH) and propose an assessment tool to measure treatment response.
Methods
Patients with superficial IH under 1 year of age were prospectively recruited between May 2012 and December 2013 at the Department of Pediatrics of Chungbuk National University Hospital. Propranolol was administered to 12 infants (median age, 3.8 months) while monitoring cardiovascular and adverse metabolic effects. If a patient showed no adverse events, the dosage was gradually increased up to 3 mg/kg/day and maintained for 1 year. We used our own scoring system to assess treatment response using parameters like change in color, and longest diameter, and thickness of the IH.
Results
Eleven out of 12 patients completed the protocol with consistent improvement of hemangiomas during therapy. Patients on propranolol showed a more than 50% involution in the first 3 months, with additional steady involution until 1 year. Patients with the highest scores at 1 month maintained their score and showed better responses until treatment termination. The patient with the lowest score at 1 month did not show any further regression and stopped propranolol treatment 4 months after initiation. In two children with recurrences after successful therapeutic regression, propranolol was effective after being reintroduced. Propranolol treatment was not interrupted in any patient due to adverse events.
Conclusion
Oral propranolol at 3 mg/kg/day showed a consistent, rapid, and therapeutic effect on superficial IHs without significant adverse events.
Introduction
Infantile hemangioma (IH) is a common benign tumor which occurs in approximately 5%-10% of 1-year-old children123). It is characterized by rapid proliferation during the first year (proliferative phase), followed by slow but inevitable involution over the next 1 to 5 years, with continued improvement up to 10 years (involuting phase)45). Although most IHs are self-limited, up to 38% of hemangiomas referred to tertiary care specialists require systemic treatment during the proliferative phase due to complications such as ulceration, bleeding, risk of permanent disfigurement, obstruction of vision, airway obstruction, or high-output cardiac failure6). Besides potential medical problems, IH may impose significant psychological distress on the patient and family.
Systemic corticosteroids have been the mainstay of treatment for complicated IH. However, side effects are commonly observed and include irritability, gastrointestinal distress, sleep disturbance, cushingoid facies, adrenal suppression, immunosuppression, hypertension, bone demineralization, cardiomyopathy, and growth retardation2). Second-line pharmacologic agents that can be used to treat problematic, proliferating IHs include interferon, vincristine, or cyclophosphamide, but these agents have significant adverse effects such as spastic diplegia, neuromyopathy, or hemorrhagic cystitis7891011).
Recently, propranolol has emerged as a promising treatment for proliferative IH12). The efficacy of propranolol for the treatment of IH was discovered by chance and first described in 2008 in 2 children who received the drug for cardiopulmonary conditions2). This finding was further supported in a study by Sans et al.13), which concluded that oral propranolol had a consistent and rapid therapeutic effect on IH. However, the treatment of IH remains empirical, and the optimal dose and duration of treatment has thus far been unexplored. Furthermore, there are no standardized scales to measure response to propranolol treatment. Therefore, we performed a prospective study to evaluate the efficacy and safety of oral propranolol to treat superficial IH measured by a combination of factors such as color, longest diameter, and thickness.
Materials and methods
1. Patients
Patients under 1 year of age with superficial IH were prospectively recruited between May 2012 and December 2013 at the Department of Pediatrics at Chungbuk National University Hospital. The diagnosis of hemangioma was determined by the characteristic clinical appearance and radiologic findings. Treatment was considered because patients' hemangiomas were associated with local complications, risk of functional or aesthetic impairment, or life-threatening locations. Patients with deep-seated hemangiomas were excluded from this study. Additional exclusion criteria included infants who had cardiovascular disorders that contraindicated propranolol use, histories of hypoglycemic episodes, recent outbreaks of wheezing, or Kasabach-Merritt syndrome. Informed consent was obtained from the parents by the respective pediatricians.
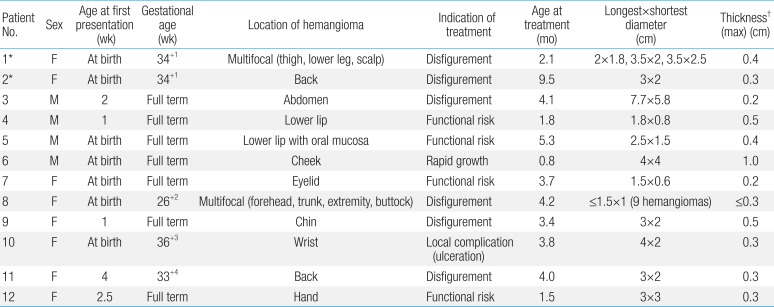
The baseline characteristics of patients receiving propranolol included each patient's gender, gestational age, age when the lesion first appeared, location, treatment indication, previous treatment, treatment duration, and clinical outcome. In addition, details regarding propranolol treatment such as dosage, age when first started and terminated, rebound, and side effects were obtained.
2. Dose and duration
Most patients were initially managed as inpatients. However, in three patients, treatment was begun in an outpatient clinic due to parental request. Patients were started on oral propranolol at 0.5 mg/kg/day in two divided doses and monitored for any cardiovascular (e.g., symptomatic bradycardia or hypotension) and metabolic side effects (e.g., hypoglycemia) over 24 hours. If the patient showed no adverse events, the dosage was increased every 24 hours up to 3 mg/kg/day (day 1, 0.5 mg/kg/day; day 2, 1 mg/kg/day; day 3, 2 mg/kg/day; day 4, 3 mg/kg/day). In cases of trouble sleeping, substitution of acebutolol was considered. Propranolol was administered for 12 months and stopped without tapering. Premature withdrawal was accepted under the following conditions: (1) if the lesion had completely involuted, (2) if there was no response at 12 weeks after initiating treatment, (3) if the patient showed no further response despite continued treatment for more than 12 weeks, (4) if the patient experienced unacceptable drug toxicity, or (5) if there was a parental request to stop the medication.
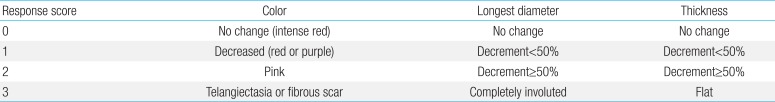
3. Evaluation
Baseline investigations before starting propranolol included evaluation of complete blood count, coagulation parameters such as prothrombin time and activated partial thrombin time, blood urea nitrogen, creatinine, electrolytes, liver function, and blood glucose. In addition, an abdominal ultrasound was included to screen visceral involvement of hemangioma. Patients' heart rate (HR) and blood pressure (BP) were monitored every 4 hours during hospitalization after the first dose of propranolol. We measured preprandial blood glucose levels at least three times a day during hospitalization and at every follow-up visit. In patients who started their treatment at an outpatient clinic, vital signs and blood glucose were obtained before initiating treatment, 2 hours, and 4 hours after treatment on the days of dose escalation. Parental guidance regarding the clinical signs of bradycardia, hypotension, and hypoglycemia was provided. Treatment tolerance was monitored by the pediatrician after each increase and at every visit. The clinical response to propranolol was evaluated 4 weeks after treatment and then at outpatient follow-up visits every 1 to 3 months. For comparison, clinical photos were taken before, during, and after treatment. Treatment response was assessed according to our own assessment tool using changes of color, longest diameter, and thickness of IH (Table 1). We measured the size of IH using a vernier caliper. Changes in size and color of the hemangioma were documented by clinical photos. Physical examination of the patients entailed documenting the size and color by one designated reviewer. In cases of multiple hemangiomas, each was evaluated separately, then we calculated the average of each score.
Results
1. Patients
Data on 12 children who received propranolol treatment are summarized in Table 2. Patients received oral propranolol at a median age of 3.8 months (range, 0.8-9.5 months). The median follow up duration was 21.1 months (range, 4.5-34.3 months). There were 8 females and 4 males. Five patients were premature. All had cutaneous lesions that appeared soon after birth (range, 0-4 weeks), and lesions were directly accessible to clinical examination without ultrasonographic assessment. Of the 12 patients, 10 patients had a solitary lesion, located on the face (n=5), back (n=2), abdomen (n=1), hand (n=1), and wrist (n=1). Two patients (patients 1 and 8) had multifocal hemangiomas. No patient had received any other prior treatment for hemangioma. Treatment was indicated due to disfigurement (n=6), functional risk (n=4), rapid growth (n=1), or local complication (n=1).
No patients had abnormal findings on baseline blood tests. All patients underwent abdominal ultrasound before treatment of IH, and no patient showed visceral involvement of hemangioma.
2. Treatment efficacy
Efficacy of propranolol treatments are shown in Fig. 1. Although the degree of response varied, all patients showed response to oral propranolol within one month after initiation of treatment. Eleven out of 12 patients completed the protocol with consistent improvement of hemangiomas during therapy. One patient (patient 5) stopped propranolol treatment prematurely due to no further response after 4 months of treatment.
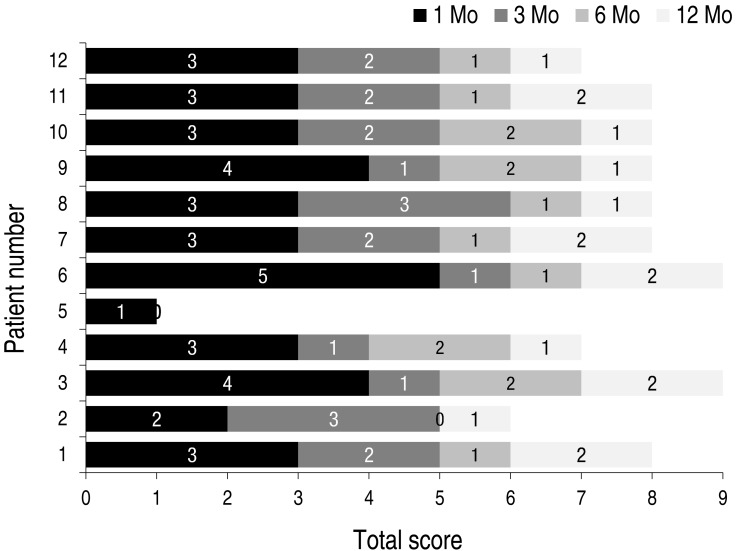
Efficacy of oral propranolol treatment for infantile hemangioma in the 12 infants enrolled. Numbers on the bar segments indicate the increase in response scores at each time point.
Among 11 patients who completed propranolol treatment with satisfactory results, 2 patients (patients 1 and 4) showed rebound growth. Relapses were mild and responded to retreatment with oral propranolol.
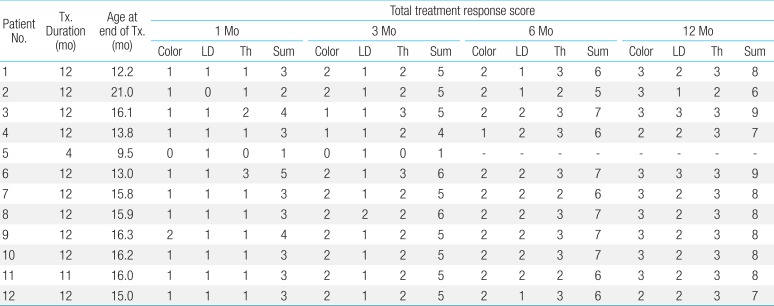
The treatment response was assessed according to our own assessment tool, which uses changes of color, longest diameter, and thickness of IH (Table 3). The median response scores (total score, 9) were 3 (range, 1-5), 5 (range, 1-6), 6 (range, 5-7) and 8 (range, 6-9) at 1 month, 3 months, 6 months, and 12 months, respectively.
Patients 1 and 2 were twins. Patient 1 had more severe lesions in terms of the number and size of hemangiomas (Fig. 2A-C) compared to her sibling, patient 2. Since her hemangiomas grew rapidly and caused disfigurement, she began to take oral propranolol at the age of 2 months and continued for 1 year (Fig. 2D-F). Two months after stopping the treatment, the hemangiomas showed rebound growth with recoloration. Patient 1 restarted propranolol treatment at her previous dose, and we observed regression of the lesions within 1 month after resuming the medication. She stopped taking propranolol after a month, and she did not experience additional recurrence.
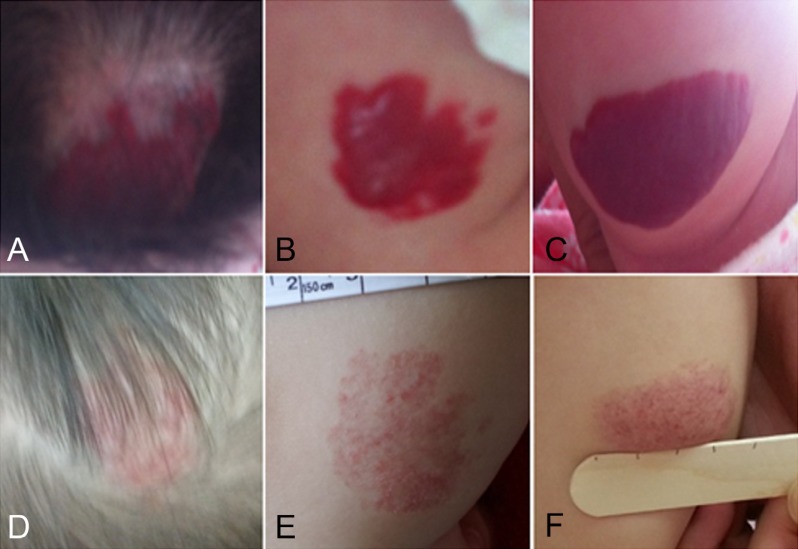
Patient 1 with multiple hemangiomas on the scalp (A), right thigh (B), and right lower leg (C). (D-F) Involution after 12 months of propranolol treatment.
Patient 4 presented with a proliferating hemangioma involving the lower lip. He was treated with propranolol for 12 months with satisfactory response. However, the patient showed recoloration with plumpness of the lip soon after cessation of propranolol. Propranolol was restarted at his previous dose, and clinical improvement involving color and size was seen within 1 week. The patient continued to take propranol for 7 months. After cessation of the second propranolol treatment, the patient showed slight recoloration with plumpness of the lip again, however, the recurrence was not progressive. Currently, he is under observation without any treatment.
Patient 5 visited our hospital due to hemangioma located on his lip and adjacent oral mucosa at birth. Oral propranolol therapy was immediately started due to concern that the hemangioma could cause the infant to have functional feeding problems. Although the diameter of hemangioma was slightly decreased with propranolol therapy, any further response to treatment was not identified for more than 3 months. Therefore, the propranolol was stopped 4 months after initiation, and the patient was lost to follow-up.
Patient 10, a 3-month-old girl, presented with ulceration of hemangioma on the right forearm with pain. Accelerated involution was observed within 1 month following propranolol therapy. The painful ulceration healed completely within 3 months after treatment.
3. Safety
No patients had difficulty in taking oral propranolol. Their vital signs, including HR and BP, were within normal limits, and their blood glucose levels and other parameters were in normal range. Propranolol treatment was not interrupted in any patients due to adverse events.
Discussion
Beta-blocker therapy is rapidly becoming the first-line therapy for IH. The mechanisms of action of propranolol on IH include direct vasoconstriction, down-regulation of angiogenetic factor or fibroblast growth factor, and up-regulation of apoptosis of capillary endothelial cells2). Propranolol is a nonselective beta adrenergic blocker; over 40 years of extensive clinical studies have not documented one case of death or serious cardiovascular morbidity as a direct effect of beta-blocker exposure14). A patient who uses propranolol can avoid the common adverse effects of prolonged use of high dose steroids. However, several well known side effects such as bradycardia, hypotension, and hypoglycemia justify close observation at the onset of treatment. In our study, there were no adverse events that necessitated a decrease in propranolol dosage or stoppage of therapy. Patient 6, the youngest patient in this study, started treatment at the age of 27 days and showed no significant adverse effects during treatment. Furthermore, no side effects related to propranolol were found in three patients who started treatment as outpatients.
Like other research groups, we have been impressed by the remarkable efficacy of beta-blockers and the rapidity of their effect on IH. In a patient with painful ulcerative hemangioma, cutaneous healing was observed shortly after initiation of treatment with propranolol. Notably, patients with high scores at 1 month kept their score and demonstrated better response until 1 year. In contrast, we prematurely stopped propranolol administration in Patient 5, who showed the lowest score at the first month of treatment due to lack of further improvement. Thus, we assumed that the initial response to propranolol could predict the final outcome of IHs.
Given the natural history of IH, a beta-blocker should be administered during the entire proliferative phase. Patients in our study were maintained on propranolol for 1 year in consideration of individual variability of the natural history of hemangiomas. Most patients experienced consistent improvement of IHs during the treatment period, except one patient (patient 5), who had a negligible early response. With propranolol, more than 50% involution of IHs was obtained in the first 3 months compared to the final status (median score 5 at 3 months; median score 8 at 1 year), with an additional steady involution until 1 year. Considering our results, 1 year of treatment does not appear to be too long to treat IHs with propranolol.
Recurrences were observed in 2 patients soon after completion of treatment. Relapses were mild and responded to re-treatment. Considering our study has relatively short follow-up duration, we need to observe whether the patients experience more relapses or not. There are some reports of recurrences of IH after treatment; however, results are heterogeneous. In the study of Sans et al.13), relapses occurred after treatment cessation before age 11 months. Denoyelle et al.15) treated their patients with a laryngeal hemangioma until age 18 months, and no relapse was seen after cessation of propranolol. Fuchsmann et al.16) reported that relapse was avoided if treatment was prolonged after involution of the lesion (age 12 months). As previously suggested, recurrences could be avoided by prolonged treatment. In our study, considering that two of eleven patients who completed propranolol treatment of 12 months showed recurrence of IH, 1-year treatment could not be enough to obtain the best outcome for some patients. In addition, abrupt cessation of propranolol could be related to recurrences. Chik et al.17) suggested that propranolol should be gradually tapered over a period of 4 weeks to avoid relapse. Similarly, Siegfried et al.18) suggested that propranolol should be gradually tapered over a period of 2 weeks. Although a potential for more frequent relapses from relatively shorter courses of treatment and a rapid taper of therapy exist, optimal treatment duration and a tapering schedule have not yet been defined. Nevertheless, patients in our study responded well to second courses of propranolol therapy.
The Hemangioma Investigator Group Research Core has developed the following two scales to measure the severity and complications of hemangioma: the Hemangioma Severity Scale, which measures the overall severity of an IH, and the Hemangioma Dynamic Complication Scale, which assigns severity grades to hemangioma complications19). These are to assess the initial status and severity of IH, not to evaluate the response of IH to treatment. The observer documented changes in color and size of the lesion on a visual analogue scale was used to assess the severity of the IH in a few studies, however, it has not been validated for IH assessment202122). In this study, we suggest our assessment tool to measure treatment response. Gaining reproducible ultrasound data of IH is challenging, depending on the size and location of the lesion and cooperation of the patient. We do not believe that ultrasonographic measurements are mandatory for cutaneous lesions, as clinical assessment of IH is relevant and accurate. Our scoring system is based on color, longest diameter, and thickness, which are key components for assessing IH, so it seems to be easy, reliable and practical for assessing superficial IH. However, further prospective studies are required to validate our scoring system.
In conclusion, oral propranolol treatment of 3 mg/kg/day for at least 1 year as first-line therapy allowed safe and rapid regression of superficial IH with less functional and esthetic impairment. More comparative, randomized studies with a greater number of patients are needed to ascertain treatment protocol along with standardized measurement instrument for IH assessment.
Acknowledgments
This study was supported by the research grant of Chungbuk National University in 2012.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.