High degree of supervision improves adherence to inhaled corticosteroids in children with asthma
Article information
Abstract
Purpose
Adherence to treatment with inhaled corticosteroids (ICS) is a critical determinant of asthma control. The objective of this study was to assess factors that determine adherence to ICS therapy in children with asthma.
Methods
Fifty-eight children with asthma, aged 5 to 16 years, used ICS with or without a spacer for 3 months. Adherence rates as measured from questionnaires and canisters, asthma symptom scores, and inhalation technique scores were assessed every 30 days. The degree of supervision by caregivers was assessed at day 30.
Results
Adherence rates measured using canisters were lower at day 60 than at day 30 (P=0.044) and did not change thereafter (74.4%±17.4% at day 30, 66.5%±18.4% at day 60, and 67.4%±22.2% at day 90). Adherence rates at days 60 and 90 and during the total study period were significantly different when measured by using questionnaires versus canisters (P<0.001, P=0.022, and P=0.001, respectively). In the comparison of adherence rates repeatedly measured at days 30, 60, and 90 and adherence rates during the total study period among the 3 groups, adherence rates in the high-degree supervision group were significantly higher than those in the low-degree supervision group (82.0±16.0 vs. 66.1±14.5, 75.4±14.4 vs. 56.2±18.4, 75.0±18.3 vs. 55.0±19.7 [P=0.027]; 77.9±12.2 vs. 59.1±11.4 [P=0.021]) after adjustment for sex and age.
Conclusion
The level of caregiver supervision is an important factor affecting adherence to ICS therapy in children with asthma. Therefore, a high degree of supervision may be required to increase adherence to ICS therapy in children with asthma.
Introduction
Asthma is a chronic inflammatory disease of the airways. Inhaled corticosteroids (ICS) are a major treatment modality for asthma. ICS decrease airway inflammation, thereby controlling the symptoms of asthma, and have fewer side effects than systemic steroids. Correct and regular use of ICS results in decreased hospitalizations and Emergency Department visits, and better quality of life (QoL) for asthma patients1).
Poor rates of adherence to ICS are common in patients with asthma, because of the long duration of treatment, difficulty of inhalation technique, and concerns about side effects2). This is a leading cause of suboptimal clinical control of the disease1). A Cochrane review of adherence to ICS in asthmatic patients found that patients took the recommended doses of medication on 20% to 73% of follow-up days, and the percentage of underuse, which was defined as taking less than 50% of the prescribed doses, ranged from 24% to 69% among adults with asthma3). In addition, Jentzsch and Camargos4) reported that adherence rates ranged from 30% to 70% in children and adolescents with asthma. These wide ranges may be caused partly by the diverse methods used to evaluate adherence rates.
ICS adherence is associated with drug-related factors, such as difficulty with inhaler devices, treatment regimens, fears about side effects, and cost of medication, and patient-related factors, such as misunderstanding of instructions, relationship with physician, poor supervision or follow-up, and underestimation of severity5). Poor adherence was associated with the use of meter-dosed inhalers (MDI) rather than dry-powder inhalers67), younger age8), level of education, number of puffs per day, and longer interval of follow-up in adults with asthma9). Lasmar et al.10) reported that in children with asthma, factors associated with adherence were the mother's level of education, replacement of the caregiver, prescription greater than two puffs per day, and the number of consultations. However, there are a few studies on factors determining adherence rate in children with asthma81112).
The aim of the present study is to assess the factors related to adherence rates to ICS in children with asthma.
Materials and methods
1. Subjects and study design
Fifty-eight children with asthma, aged 5-15 years, were recruited from the Childhood Asthma, Atopy Center in Asan Medical Center Children's Hospital, between August 2011 and October 2011. All subjects had a history of wheezing, cough, chest tightness, and/or breathlessness within the previous 12 months, and had been diagnosed on the basis of airway hyperresponsiveness, defined by a methacholine PC20 (provocative concentration of methacholine causing a 20% fall in forced expiratory volume in one second) ≤8 mg/mL. Asthma severity was defined using the National Asthma Education and Prevention Program guidelines13).
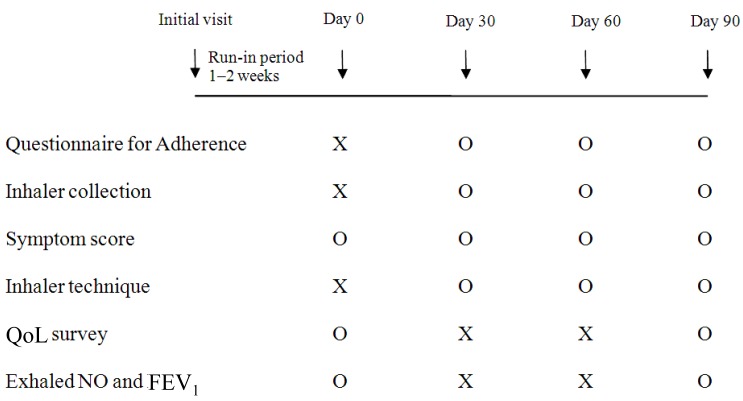
All subjects inhaled one puff twice per day of 80-µg ciclesonide (Alvesco, Takeda Pharma, Dubendorf, Switzerland) and one canister contained 60 puffs. ICS were administrated with or without a spacer (Vortex, PARI GmbH, Starnberg, Germany) fitted to the mouthpiece, depending on the age and inhaler performance. All subjects were prospectively followed up for 3 months. At baseline (day 0), subjects were educated on asthma, ICS effects, and inhalation technique by a pharmacist. Adherence rates and asthma symptom scores were assessed every 30 days by a pharmacist at the study center. Inhalation technique was checked and corrected when necessary. The degree of supervision by caregivers was assessed at day 30, through interviews. In addition, scores for QoL, levels of exhaled nitric oxide (eNO), and forced expiratory volume in 1 second (FEV1) were assessed at days 0 and 90 (Fig. 1).
Our study protocol was approved by the Institutional Review Board of the Asan Medical Center and all parents and guardians provided written informed consent following a detailed explanation of the study.
2. Adherence assessments
Adherence rates were measured by both questionnaire and canisters. Questionnaire adherence rates were reported by subjects and/or their parents at every visit. Subjects were asked to report the frequency of missed ICS inhalations during the last 30 days as zero, 1 to 4, 5 to 9, 10 to 13, or 14 per week. Questionnaire adherence rates were calculated into a percentage of inhaled puffs divided by prescribed puffs. For canister adherence rates, we educated subjects and their parents to record the day of start and end on every used canister and to use canisters sequentially. The subjects inhaled a new canister after each canister was used completely to zero puffs. At every visit, empty canisters were collected, and canister adherence rates were calculated into a percentage of used puffs estimated from the end day of the used canister divided by perscribed puffs. We classified the degree of caregiver's supervision for inhalation into 3 groups: the high-degree supervision group was defined as subjects who were given inhalations by caregivers; the low-degree supervision group was defined as subjects who administered inhalations themselves and were observed by caregivers; and the no supervision group was defined as subjects who inhaled without supervision by caregivers.
3. Asthma control assessments
We modified a previous questionnaire14) for asthma symptom scores. Patients were asked to recall their symptoms during the last month at every visit, and symptom scores were composed of wheezing, use of short-acting bronchodilator, shortness of breath, nocturnal symptoms, activity limitation, and overall asthma control. All 6 questions were scored on a 5-point scale, and a high score indicated good asthma control. In addition, QoL15) was assessed at day 0 and 90 with Korean Pediatric Asthma QoL Questionnaire, and was composed of three sections: symptomatic, emotional, and activity questions.
4. Inhalation technique assessments
Inhalation technique score measured with 6 steps and each step was scored as good, fair or poor. In MDI group, 6 steps are removing the cap from the MDI, breathing out prior to inhalation, placing mouthpieces between lips, starting to breathe in slowly, pressing down on the canister one time, keeping breathing in as slowly and deeply, and holding your breath and breathing out through the nose. In MDI with spacer group, 6 steps are removing the cap, putting the inhaler into the spacer, placing mouthpieces between lips, pressing down on the canister once, taking five breaths in the tidal volume, and cleaning a spacer in every week.
5. Measurements of lung function and atopy
Using Jaeger Masterscreen PFT (pulmonary function test) system (Jaeger Co., Wurzberg, Germany), basal lung function, including measurements of the FEV1 and forced vital capacity (FVC) was assessed, and the percentage of predicted values (% pred) for FEV1 and FVC were calculated based on previously determined reference values for healthy Korean children16).
Skin prick tests (SPTs) were performed on the children's backs by standard methods17). Commercial extracts (Allergopharma, Reinbek, Germany) of the following common allergens were used: mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae), molds (Alternaria, Aspergillus, Cladosporium, and Penicillium), pollens (grasses, trees, weeds, ragweed, mugwort, oak, beech, nettle, willow, elm, pine, hop, elder, hazel, oats, lambs quarter, ash, alder, birch, timothy, rye grass), animal dander (dog, cat) and cockroach. Histamine and isotonic saline were used as positive and negative controls. Positive SPT was defined as a mean weal diameter ≥3 mm and greater than that of the positive control, and atopy was defined by the presence of at least one positive SPT.
6. Statistical analysis
Data are presented as mean±standard deviation. The adherence rates measured by questionnaire and canister and the changes in adherence rates during the study period were compared with a paired t test. Other variables were compared between independent groups with Student t test or chi-square tests. Correlations between continuous variables were analyzed using Pearson correlation test. For the association between the degree of supervision and adherence rates, analysis of repeated measure analysis of variance was used after adjustment for sex and age. A P value of 0.05 or less was considered to be significant. IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA) was used for the analysis.
Results
1. Baseline characteristics of the subjects
Out of the 58 initially selected subjects, 31 subjects with complete adherence data were included for the analysis. Twenty-seven subjects who lost at least one inhaler canister or did not inhale prescribed canisters sequentially were excluded from the analysis. The 31 subjects included in the analysis were younger than the 27 subjects not included, and the patients used MDI with spacer were more in the included group (P=0.028 and P=0.039, respectively). In addition, those included in the analysis showed better symptoms at baseline, compared to those not included (P=0.035). Baseline characteristics of all subjects are shown in Table 1.
2. Changes in adherence during the study period
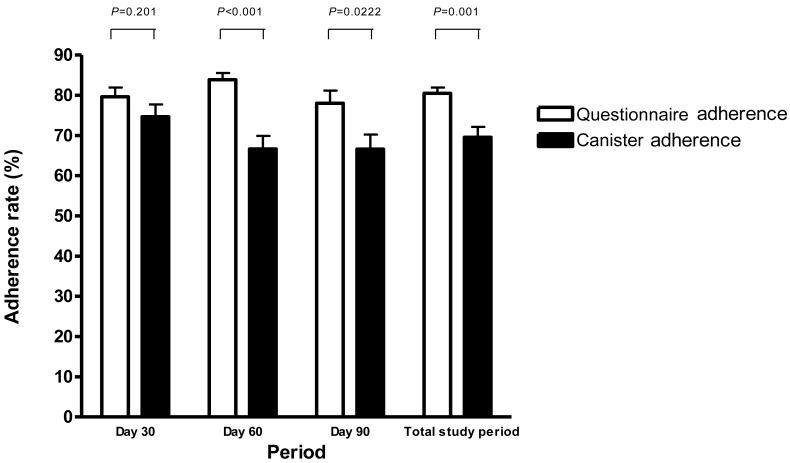
Adherence rates measured by questionnaire (questionnaire adherence) during the study period were different from those measured by canister (canister adherence). The rates of questionnaire adherence at each time point were not significantly different, and were 79.6%±12.8% at day 30, 82.8%±11.0% at day 60, 77.0%±18.4% at day 90, and 79.8%±8.7% during the total study period. However, the rates of canister adherence decreased at day 60, compared to those at day 30 (P=0.044), and not change thereafter, and were 74.4%±17.4% at day 30, 66.5%±18.4% at day 60, 67.4%±22.2% at day 90, and 69.1%±14.0% during the total study period. The adherence rate at days 60 and 90, and during the total study period, were significantly different between the two methods (P<0.001, P=0.022, and P=0.001, respectively) (Fig. 2).
3. Factors affecting adherence rates to ICS
Correlation analysis for factors affecting adherence rates was first performed with canister adherence values. Age had a marginally negative correlation with adherence rates during the total study period (r=-0.341, P=0.061). Inhalation technique scores were not significantly correlated with adherence rates. However, inhalation technique scores in subjects using spacers were significantly correlated with adherence rates at day 90 (r=0.501, P=0.021) and during the total study period (r=0.573, P=0.008), while there was no association between adherence rates and technique scores in subjects using metered dose inhalers without spacers. Adherence rates were not significantly associated with sex, educational levels of caregiver, economic status, severity of asthma, symptom scores, QoL scores, inhaler type, and caregiver at day 0.
Adherence rates in the high-degree supervision group were 82.0%±16.0% at day 30, 75.4%±14.4% at day 60, 75.0%±18.3% at day 90, and 77.9%±12.2% during the total study period. Adherence rates in the low-degree supervision group were 66.1%±14.5% at day 30, 56.2%±18.4% at day 60, 55.0%±19.7% at day 90, and 59.1%±11.4% during the total study period. Adherence rates in the no supervision group were 72.5%±18.3% at day 30, 64.4%±18.3% at day 60, 66.1%±19.9% at day 90, and 68.0%±13.9% during the total study period. In the comparison of adherence rates repeatedly measured at day 30, 60, and 90 among the three groups, adherence rates in the high-degree supervision group were statistically significantly higher than those in the low-degree supervision group after adjustment for sex and age (P=0.027). In addition, adherence rates during total study period in the high-degree supervision group were statistically significantly higher than those in the low-degree supervision group after adjustment for sex and age (P=0.021). Adherence rates of the no supervision group at every visit and during the total study period were between those of the high-degree and low-degree supervision groups without significant differences (Table 2).
Questionnaire adherence rates did not show any correlations with the factors above (data not shown).
4. Reasons for poor adherence by questionnaires
Subjects or caregivers were asked the reasons for poor adherence by questionnaire at every visit (93 responses from 31 subjects at 3 visits). The reasons were as follows: forgetfulness of inhalation (74 of 93, 78.7%), underestimation of asthma severity (8 of 93, 8.5%), poor supervision (6 of 93, 6.4%), children's dislike of inhaled medication (3 of 93, 3.2%), and fears about side effects of ICS (2 of 93, 2.1%).
Discussion
There are several ways to quantify adherence to ICS in asthma patients, including self/parental reports, pharmacy records, measurement of canister weight, and electronic devices. The rates of adherence vary depending on the method of assessment. Whereas electronic devices are regarded as one of the most accurate methods, adherence reported by self/parents was more likely to be overestimated than the other methods1218192021). Studies comparing adherence rates measured by different methods showed that adherence rates reported by self/parent were 80%-97%, those measured by canister weight were 51%-69%, and those measured by electronic device were 46%-50%1218). The method of calculating adherence rates by measuring a decrease of canister weight could be distorted by episodes of canister dumping, or expelling medications into the air, before evaluation. However, because the difference between adherence rates measured by canister weight and electronic device was relatively low, previous studies suggested that adherence rates measured by canister weight could be comparable to those obtained by electronic monitoring182223).
We measured adherence rates by collecting canisters and calculating a percentage of used puffs per month estimated from the time to empty canister during study periods instead of measurement of canister weight. Similar to findings of other studies, our results showed a significant difference between adherence rates measured by self/parents reports and canister1218192021). Adherence rates decreased at 60 days after the start of ICS treatment, and did not change thereafter.
Adherence rates to ICS tend to decrease over time as reported by other studies102425). Jentzsch et al.18) reported that in asthmatic children adherence rates decreased from 58.3% to 37.9% as measured by electronic device, and from 100.0% to 96.4% as measured by self/parents reports throughout 12 months of follow-up. In the present study, adherence rates measured by canister decreased at day 60, and did not change thereafter. However, the rates measured by questionnaire did not significantly change over time. These results suggest that the clinician should regularly check adherence rates by an appropriate quantitative method.
Our study found that adherence rates to ICSs in asthmatic children correlated with the degree of supervision, and in the comparison of three groups, those with a high-degree of supervision had better adherence than those with a low-degree of supervision. However, adherence rates of no supervision group were not significantly different from those of the other groups. The tendency that the low-degree supervision group did not have better adherence than no supervision group might be caused by the difference of age. The mean age of no supervision group (9.8±3.0) was older than those of the low-degree supervision group (7.8±2.4, P<0.05). Older children might need the less support of caregiver.
This is the first study to demonstrate that the degree of supervision can be an important factor in determining adherence in children with asthma. Previous studies reported that adherence to ICS was related to children's age812), the frequency of the prescribed regimen1011), the interval of follow-up10), the type of ICS device7), care givers26), and mother's educational level10) in children with asthma. Because ICS medication intake in children is dependent on their parents and caregivers, their role is important in adherence to ICS26). Caregiver-related factors have been reported to be familial instability12), parental stress11), replacement of caregiver10), and attendance of day-care center20).
In addition, instruction on inhalation technique has been reported to improve both inhalation technique scores and adherence rates to the therapeutic regimen in asthma8). In our study, inhalation technique scores in subjects using spacers were correlated with adherence rates. Correct inhalation technique, resulting from the caregiver's beliefs and motivation, may be related with adherence to ICS. However, there was no association between adherence rates and technique scores in subjects using metered dose inhalers without spacer, therefore technique score was not adjusted for in the final model.
Low adherence is related to inadequate asthma control, demonstrated by increased numbers of asthma exacerbations and visits to emergency departments, and worsening of symptom scores and QoL8). Lasmar et al.27) reported that in 122 asthmatic children there were significant differences in adherence rates to ICS in those who maintained asthma control compared to those who did not (84% vs. 47% in the 12 months), and that optimal asthma control required adherence rates higher than 80%. In our results, high-degree supervision kept adherence rates at almost 80%, although adherence rates of the low-degree supervision group were almost 60%. However, adherence rates at every visit did not correlate with symptom scores, the levels of FEV1 and eNO (data not shown). The discrepancy between the results of our and other studies could be caused by the severity of asthma, distribution of adherence rates, and duration of study period. The severity of most patients was mild persistent in our study and the distribution of adherence rates was skewed to relatively better adherence than other studies.
This prospective study had a high drop-out rate, but factors determining adherence rates were adjusted for age, which was different between subjects included and not included for analysis. In the present study, repeated interviews and inhalation technique assessments were provided by a pharmacist. Repeated advice given by a pharmacist, such as the role of ICS and their necessity for control of symptoms, and addressing the patient's concerns about ICS, might further promote adherence to the treatment regimen as demonstrated by other studies828). High adherence rates in our study, compared to other studies, might be caused by this effect. In addition, we assessed the degree of supervision at day 30, however this degree also could be changed by education during the period of study.
In conclusion, the degree of caregiver's supervision is an important factor affecting adherence to ICS in children with asthma. Therefore, a high degree of supervision may be required to increase adherence to ICS in children with asthma.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.