Clinical outcome of acute necrotizing encephalopathy in related to involving the brain stem of single institution in Korea
Article information
Abstract
Purpose
Acute necrotizing encephalopathy (ANE) is a fulminant disease of the brain characterized by bilateral thalamic lesions, and is prevalent among children in East Asia. The prognosis of ANE is usually poor with a high mortality rate and neurological sequelae. This study aimed to delineate the clinical characteristics and prognostic factors of ANE.
Methods
We retrospectively analyzed clinical data of 399 pediatric patients with encephalitis who were admitted to Samsung Medical Center from December 1998 to March 2011. We enrolled ten patients (11 cases) with ANE and analyzed their demographic, clinical, and neuroimaging data. The location and extent of the brain regions were checked based on fluid-attenuated inversion recovery, T1-, and T2-weighted imaging findings; the presence of contrast enhancement, restricted diffusion, and hemorrhage.
Results
Ten patients were identified, including one patient with two episodes. The median age of onset was 1.5 years (0.4-8.4 years). The mortality rate was 40%, and only 30% of patients survived without neurological sequelae. The definite involvement of the brainstem on brain magnetic resonance imaging was significantly correlated with mortality (P=0.04).
Conclusion
Broad and extensive brainstem involvement suggested the fulminant course of ANE. Early diagnosis of ANE before brainstem involvement, through careful identification of symptoms of brain dysfunction, may be the best way to achieve better neurological outcomes.
Introduction
Acute necrotizing encephalopathy (ANE) is a novel disease entity proposed by Mizuguchi1) in 1995 characterized by rapid progression of brain dysfunction and disastrous outcom. It occurs in healthy children after a common febrile illness with typically selective symmetric involvement of the thalamus, brain stem, and cerebellum1,2,3). ANE has been reported worldwide, but the condition seems more prevalent in East Asian countries such as Japan, Taiwan, and Korea4,5,6,7). The reported clinical course of ANE is fulminant with rapid deterioration of consciousness, convulsion and brainstem dysfunction. The mortality rate of ANE has been reported to reach about 30%, and most of the affected children die in the first week6,7,8,9,10,11). Survivors of ANE suffer from mild to moderate neurologic sequelae, and less than 10% of patients recover completely8,12).
There have been a few studies regarding prognostic factors of ANE such as early steroid treatment, magnetic resonance imaging (MRI) findings, or involvement of the brain stem2,8,12,13). However, additional data are still needed to firmly determine prognostic factors of ANE. In this study, we analyzed the demographic, clinical, laboratory, and radiologic data of ten Korean children with ANE at a single institution to find out prognostic factors of ANE.
Materials and methods
1. Patients
We reviewed the medical records of 399 pediatric patients (≤18 years old) with encephalitis who were admitted to Samsung Medical Center (Seoul, Korea) from December 1998 to March 2011. This study included patients who diagnosed with ANE based on the criteria proposed by Mizuguchi1), acute encephalopathy following viral disease with rapid deterioration of consciousness, absence of cerebrospinal fluid (CSF) pleocytosis, increase in CSF protein, neuroimaging findings of symmetric brain lesions involving the bilateral thalami, elevated serum aminotrasferase to a variable degree, no increased blood ammonia, and clinical absence of other diseases resembling ANE. We exclude other resembling diseases, suggested by that Mizuguchi1), such as overwhelming bacterial and viral infections, fulminant hepatitis, Reye syndrome, other toxin induced disease, Leigh encephalopathy and related mitochondrial cytopathies, Wernicke encephalopathy, acute disseminated encephalomyelitis or other types of encephalitis, vasculitis, cerebral infarction, and so on.
2. Clinical and radiologic data
In each episode, clinical data were collected based on the retrospective chart review including sex, age, initial clinical manifestations, clinical course, initial laboratory findings, electroencephalography (EEG) findings, causative organism of associated febrile illness, treatment modalities, and outcome.
Neuroimaging was reviewed by a neuroradiologist. The location and extent of signal abnormalities were checked by brain computed tomography (CT) or MRI with fluid-attenuated inversion recovery (FLAIR), T1-, and T2-weighted images. The presence of contrast enhancement in the lesions was also identified. For differentiation of cytotoxic and vasogenic edema of the lesions, diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) map images were used. Vasogenic edema (interstitial edema) usually shows high-signal intensity on DWI with an increased ADC map. However, cytotoxic edema (cellular swelling) expresses high-signal intensity on DWI and low-signal intensity on ADC maps. Detection of hemorrhage in the lesion was aided using T2- and T1-weighted images and gradient-echo imaging in patients with MRI. Neuroimaging findings were analyzed by the distribution, pattern and presence of hemorrhage. We paid particular attention to the analysis of the extent and signal intensity of brain stem lesions, the midbrain, pons, and medulla oblongata, for the identification of prognostic factors. The findings of brain stem involvement were as follows: diffuse broad hypointensity on CT, broad hyperintensity on T2-weighted/FLAIR MRI, diffusion restriction (cytotoxic edema) on DWI/ADC, and hemorrhage. The brain stem lesions were divided into three groups (none, mild, and definite) based on extent and signal-intensity as followed: none defined as absent involvement, mild defined as extent of involvement in below 2 lesions among midbrain, pons, and medulla oblongata and subtle signal change without diffusion restriction and hemorrhage, and definite defined as broad extent more than three or marked signal change with diffusion restriction or hemorrhage.
Ethical approval for this retrospective study was provided by the Institutional Review Board of Samsung Seoul Medical Center in Seoul, Korea (SMC 2011-04-104-002).
Data are presented as median and range. Statistical analysis was performed to identify different factors between death and survival groups with the Fisher exact test. And the predictors of mortality in ANE was estimated using multivariate Cox regression and Mann-Whitney test with PASW Statistics 18.0 (SPSS Inc., Chicago, IL, USA). The statistical significance threshold was set at P<0.05 corrected.
Results
1. Patient characteristics and clinical presentation
Of the 399 pediatric patients, we identified ten patients (male:female=6:4) with ANE, including a patient with two episodes (Table 1). The median age of onset was 1.5 years (0.4-8.4 years). Family history of ANE was unremarkable in all patients. None had any recent immunization.
The clinical characteristics of the patients and the clinical presentations of viral illness are summarized in Table 1. Prior to the onset of ANE, nine patients showed fever, and all children had symptoms of viral illness; upper respiratory tract infection (n=6), acute gastroenteritis (n=7) and acute pharyngo-tonsillitis (n=2). The median body temperature was 38.5℃ (37.5℃-40℃) during the antecedent febrile illness. The first sign of encephalopathy appeared after a median of four days (1-9 days) from the onset of antecedent infection (Table 2). Altered mental status was observed in all cases: drowsiness (n=10, including recurrent case) and confusion (n=1). Nine patients (90%) had seizures (generalized seizure, n=5; complex partial seizure, n=4). They were managed with various anticonvulsants (phenytoin, n=8; phenobarbital, n=5; topiramate, n=1; carbamazepine, n=1; levetiracetam, n=1). The seizures occurred in the initial phase when the deterioration of consciousness appeared. The seizures were well controlled with antiepileptic drugs without recurrence during hospitalization. Progression of encephalopathy to maximum level of mental status deterioration occurred between two to twelve days after the initial vial illness. The lowest level of consciousness ranged from drowsy to coma (Table 2).
2. Neuroimaging
The neuroimaging findings are summarized in Table 3. Eight patients were evaluated with both brain CT and MRI. However, two patients (patients 1 and 9) underwent only brain CT, because they were clinically too unstable to have brain MRI. The abnormalities in brain MRI were most well defined by T2-weighted images and FLAIR sequences (Fig. 1).
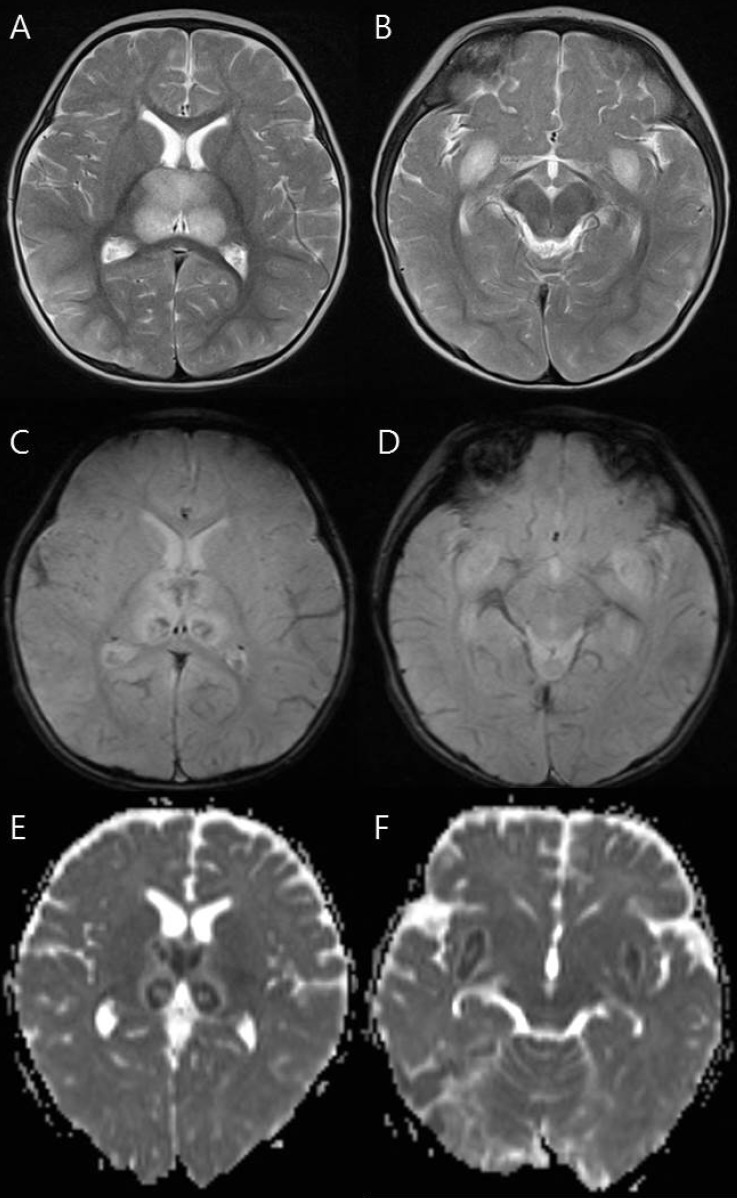
Magnetic resonance imaging findings of a 9-month-old girl (patient 7) who died. (A, B) T2-weighted axial images show increased signal intensity in the thalami and medial temporal lobe. (C, D) Axial gradient-echo images show decreased signal intensity in the thalami and medial temporal lobe indicating hemorrhage. (E, F) Apparent diffusion coefficient images reveal hypointensity in the thalami and medial temporal lobe.
Every patient showed symmetric involvement of the thalamus, however, patient 6 showed bilateral but asymmetric (left>right) thalamic involvement (Fig. 2). She had a milder clinical course (confused mental status and intensive care unit [ICU] care for only two days), and completely recovered without neurologic sequelae and no residual lesions on follow-up brain MRI.
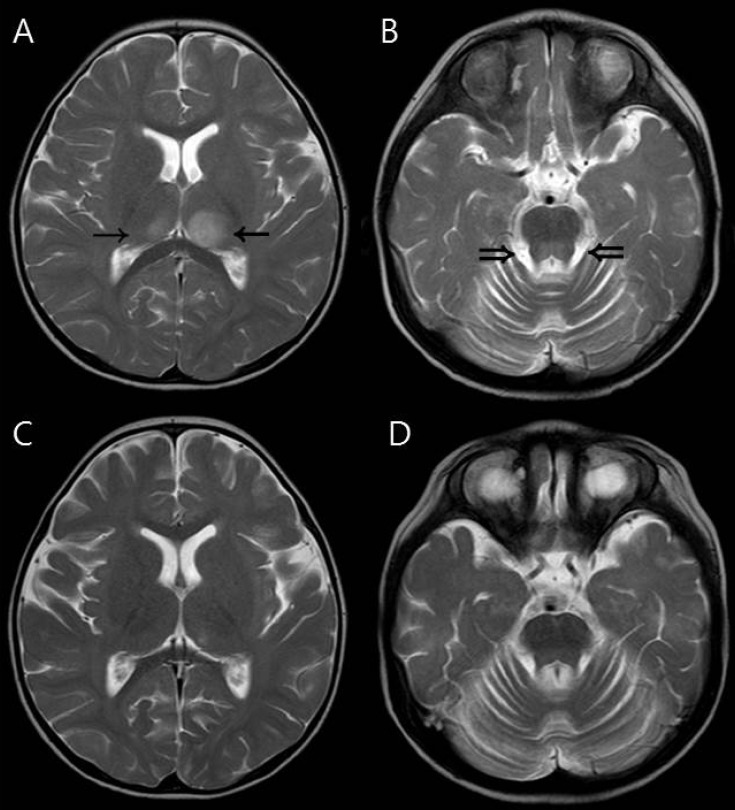
Magnetic resonance imaging findings of a 19-month-old girl (patient 6) who had asymmetric (left dominant) thalamic involvement and recovered completely. (A, B) Initial T2-weighted axial image showing asymmetric increased signal intensity in the thalami (arrows). The dorsal pons (double arrows) was also involved. (C, D) These abnormal signal intensities disappeared on follow-up magnetic resonance images obtained one week after the initial study.
Cervical spinal cord lesions were observed in patient 2 (Fig. 3). He had extensive involvement of the brain including the bilateral thalami, basal ganglia, broad brain stem and cerebellum.
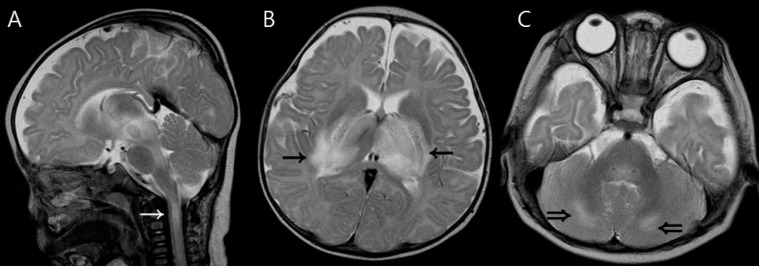
Magnetic resonance imaging findings of a 5-month-old boy (patient 2) who had upper cervical spinal cord involvement. (A) T2-weighted sagittal image shows a high signal intensity lesion in the upper cervical spinal cord (white arrow). (B, C) T2-weighted axial images show increased signal intensity in the thalami (arrows) and cerebellum (double arrows).
Seven cases (63.6%) had brain stem involvement. We analyzed the extent (the midbrain, pons, and medulla oblongata) and signal-intensity of brain stem lesions. The extent and signal-intensity of brain stem lesions were divided into three groups: absent signal change (none, n=4), subtle signal change (mild, n=3), and broad extent and marked signal change (definite, n=4). The findings of brain stem involvement were as follows: diffuse broad hypointensity on CT, broad hyperintensity on T2-weighted/FLAIR MRI, diffusion restriction (cytotoxic edema) on DWI/ADC, and hemorrhage.
3. Electroencephalography
All patients underwent EEG during acute encephalopathic periods. Ten cases (10/11, 91%) initially showed abnormal EEG. The EEG revealed diffuse encephalopathic findings characterized by diffuse background slow activity (n=6) or voltage suppression (n=3) on cerebral background rhythm. In addition, regional intermittent slow (n=1) and focal epileptiform activities (n=3) were observed. EEG seizure was recorded in two patients (patient 3 and 10).
4. Laboratory data
Mild elevation of liver transaminases was found in ten cases. The median level of aspartate aminotransferase was 53 U/L (27-209 U/L), and the median level of alanine aminotransferasewas 31 U/L (15-242 U/L). The metabolic tests such as serum amino acid analysis, serum ammonia and lactate/pyruvate level, and urine organic acid analysis were all normal. Lumbar puncture was performed in eight patients (73%); cerebrospinal fluid (CSF) pleocytosis (>5/L) was found in four patients (9-175/µL), and increased CSF protein (>45 mg/dL) was found in five patients (88-246 mg/dL). Pathogens responsible for antecedent infections were identified in 63.6% of cases (7/11) (Table 1).
5. Treatment
The clinical course and management during their hospital admission are summarized in Table 3. All patients were admitted to the pediatric ICU, but patient 5 was cared in the general ward for the second episode. Patient 1 received unusual long term ICU care for 230 days after the diagnosis of brain death, because the parents wanted continuous care until cardiac death. The median duration of ICU stay in nine patients except patient 1 was eight days, from 1 to 15 days. Until the patients were diagnosed as having ANE with brain imaging and were ruled out of other possible causes, we provided treatment for possible viral encephalitis or bacterial meningitis such as vancomycin, third-generation cephalosporin, or acyclovir. We also prescribed oseltamivir to patients who had positive results for Influenza A or B. Intravenous immunoglobulin (IVIG, total 2 g/kg for 2-5 days) and high-dose steroids (methylprednisolone 15-30 mg/kg/day for 3 or 5 days) were used to treat ANE: both intravenous IVIG and high-dose steroid therapy (n=6), IVIG only therapy (n=1), and high-dose steroid therapy (n=2). These patients started treatment within 24 hours after diagnosis with ANE. Two patients (patient 3 and 9) were not treated with IVIG or high-dose steroid therapy. Patient 3 was transferred to our hospital in a comatose state and was confirmed the diagnosis of brain death the next day. Therefore, we did not try either IVIG or high dose steroids on the patient. The patient died two weeks after conservative management. Patient 9 was also transferred to our hospital in a comatose state, and he was declared brain death on the third day of admission and died on the fifth hospital day.
6. Outcome and prognostic factors
The overall mortality rate was 40% (4/10). Three patients became brain death within three days from the day of mental status change. The median follow-up duration of six survivors was 3.3 years (range, 1.6-5.6 years). Neurological impairments such as motor deficits, developmental language delay and epilepsy were observed in three of six survivors. Definite involvement of the brain stem in brain imaging exhibited a significant statistical association with mortality (P=0.041) by multivariate Cox regression and Mann-Whitney test. As stated above, patients had no sooner started the IVIG or high-dose steroid therapy than they diagnosed with ANE. Therefore, the length of time between symptom onset and treatment was almost equal to that from symptom onset to diagnosis. There were no statistical associations between mortality and the factors studied; age, duration from symptom onset to diagnosis and treatment, causative agent of antecedent infection, brain hemorrhage and use of steroids or IVIG (Table 4).
Discussion
This study delineated the clinical characteristics, various disease courses and prognosis of ANE patients at a single institution and revealed that definite involvement of the brain stem noted by brain imaging is a significant prognostic factor of ANE.
ANE in childhood is rare. It is a fulminant and life-threatening neurologic disease that usually occurs in healthy children. The reported mortality rates are as high as 30%1,13). In this study, the mortality rate was 40% which is similar to that of previous studies. Severe neurologic sequelae have been reported in 15%-35% of survivors, such as intention tremor, ataxic gait, hemiparesis, quadriplegia, athetosis, dysarthria, abnormalities of extraocular motility, and mental slowing1,6,13). In the present study, three of six survivors showed neurologic sequelae such as motor deficits and language developmental delay.
There have been many studies to identify prognostic factors associated with ANE. According to previous studies, age, sex, and the presence of causative agent were not significant prognostic factors, which are consistent with the findings in this study1,14,15). In 2009, Okumura et al.16) evaluated the relation between outcome and treatment with steroids or IVIG in 34 children with ANE. They advocated that IVIG is not correlated with outcome but steroid treatment within 24 hours after the onset was related to the better outcomes in patients without brainstem lesions. In this study, however, steroid treatment within 24 hours of symptom onset was not associated with outcome.
In the present study, definite radiologic involvement of the brain stem was significantly associated with mortality. The relationship between characteristic MRI findings and outcome has also been reported in several previous studies. Kim et al.6) reported that the presence of hemorrhage and localized atrophy in MRI is a significant prognostic factor. Moreover, some studies showed that involvement of extra thalamic lesions such as the brain stem is more correlated with poor clinical outcomes16). Wong et al.8) documented positive correlations between: clinical outcome and the MRI score; and the location of brain stem and/or white matter involvement and the presence of hemorrhage and/or cavitation. Unlike previous studies, we evaluated the relationship between the severity and extent of brainstem lesions and mortality. In this context, a broad extent and a definite signal change of brainstem lesions were significantly correlated with mortality. We therefore suggest that involvement of the brainstem is a sign of a profound progression of the disease. Extensive brainstem lesions with extensive damage in the medullar and pons were followed by severe brainstem dysfunction characterized by respiratory insufficiency, sudden cardiopulmonary collapse and neurogenic pulmonary edema, all of which could explain the high mortality rate of this disease. In our study, steroid treatment in those patients with a broad extent and a definite signal change of the brainstem resulted in no improvement in outcome. We did not observe a statistical correlation for treatment start time.
There was a patient with cervical spinal cord involvement (Fig. 3). This patient's brain MRI revealed extensive involvement of the deep cortex, brain stem, cerebellum, and upper cervical cord. The lesion was discrete and confined to the C1 and C2 cervical spine. He showed decreased motor power in both upper extremities. We suspected that spinal cord involvement is possible in children with severe extensive ANE. There has been only one report about spinal cord involvement in ANE17). That case had clinical symptoms of spinal cord lesions, such as acute urinary retention, but revealed reversible favorable outcome. Contrary to our case, the extent of ANE in that case was limited to the bilateral thalami and frontal periventricular white matter. Evaluation of the spinal cord is recommended for adequate management of patients who show spinal symptoms or extensive cerebral lesions.
Most cases of ANE are sporadic and do not recur. However, familial and recurrent cases have been reported, and some of them were linked to mutations in RAN-binding protein2 (RANBP2)13,18,19). Recurrent or familiar ANE is considered an abnormal immune response, a cytokine storm that is triggered by an environmental stimulus in genetically susceptible individuals. In our study, patient 5 experienced recurrent episodes but not disease relapse. We performed the RANBP2 mutation test on patient 5. However, we did not find any mutations in the coding region of RANBP220).
This study has a limitation stemming from the rarity of the disease. Patients were treated at a single institution but managed over a long time period, under different clinical situations.
In conclusion, definite involvement of brainstem on brain MRI was associated with the fulminant course of ANE. Early diagnosis and prompt treatment of ANE before brainstem involvement through the close observation of symptoms of brainstem dysfunction in patients with mental status change after a febrile illness may ensure the better neurological outcome.
Notes
No potential conflict of interest relevant to this article was reported.



