Efficacy of imatinib mesylate-based front-line therapy in pediatric chronic myelogenous leukemia
Article information
Abstract
Purpose
Despite the established role of imatinib (IM) in chronic myelogenous leukemia (CML) in adults, there are few reports on its efficacy in children. In this study, we compared the outcomes of children with CML before and after the advent of IM-based treatment.
Methods
The study cohort consisted of 52 patients treated for CML at the Department of Pediatrics, The Catholic University of Korea from January 1995 to October 2010. Patients were divided and analyzed according to the preImatinib group (pre-IMG) and imatinib group (IMG).
Results
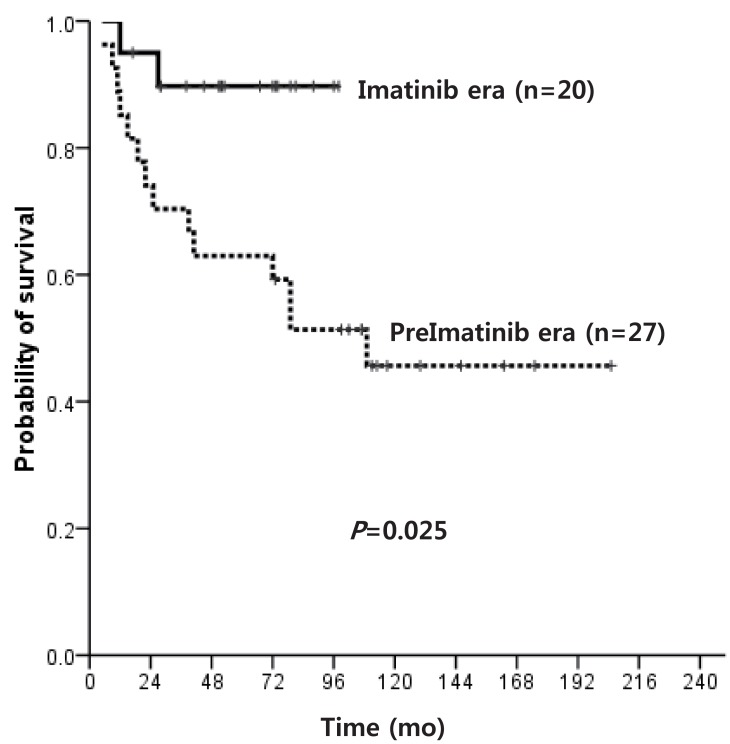
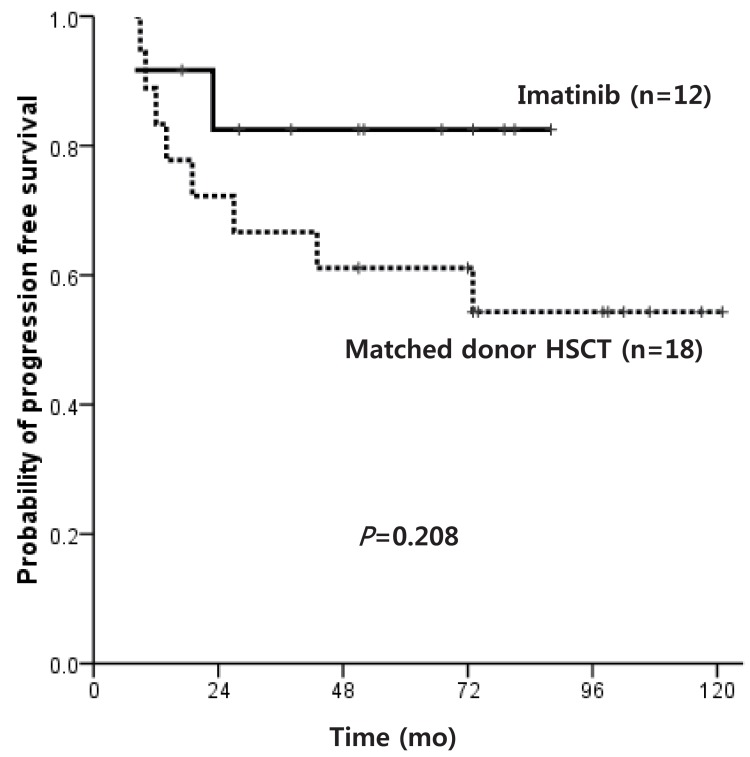
Median age at diagnosis for the overall cohort (pre-IMG, n=27; IMG, n=25) was 9 years, with a median follow-up duration of survivors of 84 months. Except for 5 patients in the IMG, all were diagnosed in chronic phase (CP). The overall survival (OS) of patients diagnosed in CP was 45.7% and 89.7% for pre-IMG and IMG, respectively (P=0.025). The OS of hematopoietic stem cell transplantation (HSCT) recipients in the 2 groups was similar, but the OS of patients diagnosed in CP who did not receive HSCT was superior in IMG (91.7% vs. 16.7%, P=0.014). Of the 12 patients in IMG who remained on IM without HSCT, 2 showed disease progression, compared to 11 of 12 in pre-IMG. No difference was observed in the progression free survival (PFS) of matched donor HSCT recipients and IM-based treatment recipients.
Conclusion
Similar PFS of patients treated with IM and those who received matched donor HSCT underscore the potential of IM as effective first-line treatment in childhood CML.
Introduction
Chronic myelogenous leukemia (CML) is a clonal myeloproliferative disorder characterized by the presence of the Philadelphia chromosome arising from the reciprocal translocation between the long arms of chromosomes 9 and 22, resulting in the BCR-ABL chimeric gene that expresses an abnormal fusion protein with altered tyrosine kinase activity1).
CML is diagnosed mostly in adults, with 10% of patients consisting of children, and comprising 3% of overall pediatric leukemia incidence2). Past treatment included hydroxyurea, busulfan, and interferon-α (IFN-α) as means of controlling the disease, with allogeneic hematopoietic stem cell transplant (HSCT) offered as the only curative treatment option3). However, since the introduction of imatinib mesylate (IM; STI571, Gleevec or Glivec), a BCR-ABL tyrosine kinase inhibitor (TKI), IM has replaced IFN-α or HSCT as first-line treatment of adult CML patients diagnosed in chronic phase (CP)4,5). In adults, recent trials have tested the feasibility of stopping imatinib altogether in patients with continued molecular remission, underscoring the possibility of IM-based cure of CML6), and the possibility of TK-based cure of disease has become greater with introduction and expanding use of more effective second generation TKIs7,8).
However, studies of TKI use in children are few, and the role of TKI in the overall treatment schema of childhood CML has not been established. Recently, several studies have been published concerning TKI use in children, with high overall survival (OS) and progression free survival (PFS) reported9-11). On the basis of these studies, IM has been advocated as first-line treatment of childhood CML in CP, although reports on IM use in children are still too few, and questions such as the duration of TKI treatment necessary, and potential complications resulting from long-term TKI use remain unaddressed12). Furthermore, second generation TKIs have had limited testing in the pediatric setting, mostly in the form of clinical trials13). Hence, whether TKI-based treatment can fully replace HSCT in the treatment of CML CP, as is the case with adult patients, requires further, long-term investigation in the pediatric setting.
In this study, we attempted to identify the role of IM as possible front-line treatment for childhood CML by analyzing outcomes in a single institution cohort of patients before and after the introduction of IM.
Materials and methods
1. Study cohort
Patients treated for CML at the Department of Pediatrics, The Catholic University of Korea from January 1995 to October 2010 were included in the cohort. Retrospective analysis up till June 2012 was done. Overall, 52 patients were included in the cohort, with a median follow-up duration of survivors of 84 months (range, 17 to 205 months).
Age at diagnosis, gender, clinical symptoms and signs, peripheral blood (PB) and bone marrow (BM) findings, and cytogenetic and molecular findings were investigated. Diagnosis was based on PB and BM findings, and identification of the Philadelphia chromosome through conventional cytogenetic study and fluorescent in situ hybridization, with BCR-ABL fusion gene transcript level checked using quantitative real time-polymerase chain reaction (qRT-PCR). The accelerated phase (AP) or blastic crisis (BC) was defined according to World Health Organization criteria14).
The study cohort was divided into PreImatinib group (pre-IMG) and imatinib group (IMG) according to whether IM was used as front-line treatment. Outcomes were analyzed according to whether HSCT was administered for treatment.
In pre-IMG, patients received hydroxyurea, etoposide, and/or IFN-α as means of disease control before allogeneic transplantation which was considered the final curative treatment modality in all patients. All patients in IMG received IM as initial treatment, with a dosage of 260 mg/m2/day for patients in CP and 340 mg/m2/day for patients in advanced disease phase.
2. Treatment response evaluation
Treatment response was assessed according to European Leukemia Net guidelines15). A complete hematologic response (CHR) was defined as WBC<10×109/L, platelets<450×109/L, basophils<5% and no immature myeloid cells in PB, and no splenomegaly. Cytogenetic response (CyR) was assessed based on a minimum of 20 metaphase preparations and was defined as complete CyR (CCyR, 0% Philadelphia chromosome-positive [Ph+] metaphases), partial CyR (pCyR, 1-35% Ph+ cells), minor CyR (mCyR, 36-65% Ph+ cells), or minimal CyR (minCyR, 66-95% Ph+ cells). Molecular response (MR) was assessed by qRT-PCR, and was graded as complete molecular response (CMR, undetectable BCR-ABL mRNA transcripts) and major MR (MMR, ≤0.1% on the international scale compared with the baseline level).
Progression was defined as progression to AP or BC or death during follow-up. Side effects of IM were assessed according to National Cancer Institute criteria (Common Terminology Criteria for Adverse Events ver. 4.0 [U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Bethesda, MD, USA)16).
3. Statistical methods
OS, PFS, and time to events were calculated from the date of diagnosis and HSCT, respectively, by the Kaplan-Meier method. Differences in survival curves were compared using the log-rank test. Comparisons among groups were performed using chi-square test and Fisher exact test for categorical variables, and Wilcoxon 2-sample test for continuous variables. All statistical tests of significance were two tailed and analyses were performed using software SPSS ver. 16 (SPSS Inc., Chicago, IL, USA). For all calculations, a P value <0.05 was considered significant.
Results
1. Patient characteristics
Median age at diagnosis was 9 years (range, 2 to 15 years), and 47 of 52 patients (90.4%) were diagnosed in CP. The complete blood count showed leukocytosis (median, 227.2×109/L), anemia (median, 9.2 g/dL), and thrombocytosis (median, 557.2×109/L). Pre-IMG and IMG consisted of 27 and 25 patients, respectively, with 5 patients in advanced disease states (1 AP, 4 BC) all found in IMG. Except for anemia, no significant clinical difference was found between the 2 groups (Table 1).
2. Treatment and clinical outcome
Details of the treatment and clinical course of patients in both Pre-IMG and IMG are shown in Fig. 1. The OS of pre-IMG and IMG was not significantly different, 45.7% and 78.4%, respectively (P=0.149). However, the OS of patients diagnosed in CP only was significantly higher in the IMG (89.7% vs. 45.7%, P=0.025) (Fig. 2).
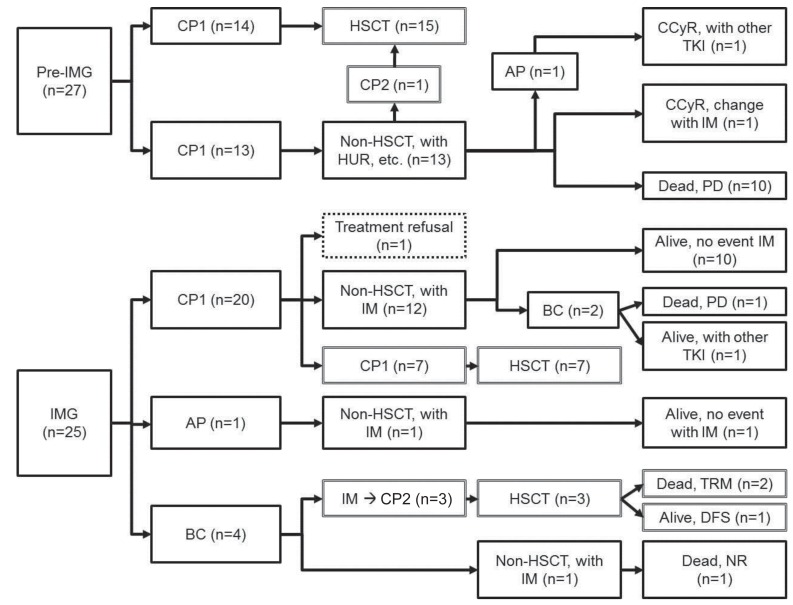
Treatment and clinical outcome of the patient cohort. Pre-IMG, pre-imatinib group; CP1, first chronic phase; HSCT, hematopoietic stem cell transplantation; CP2, second chronic phase; HUR, hydroxyurea; AP, accelerated phase; CCyR, complete cytogenetic response; TKI, tyrosine kinase inhibitor; IM, imatinib; PD, progression of disease; IMG, imatinib group;BC, blastic crisis; TRM, treatment related mortality; DFS, disease free survival; NR, no response.
1) Allogeneic HSCT
Fifteen patients in the pre-IMG (55.6%) and 10 in the IMG (40%) received HSCT. Median age at transplantation was 9 years and median time from diagnosis to transplant was 9 months (Table 2). All patients received transplant in CHR, with 1 patient in pre-IMG and 3 patients in IMG (diagnosed in BC) receiving transplant in second CP (CP2). A matched sibling donor (MSD) was the most common donor type (13 patients: 6 pre-IMG, 7 IMG), followed by matched unrelated donor (7 patients: 5 pre-IMG, 2 IMG). Cord blood was the cell source for all 3 mismatched unrelated donor transplants (pre-IMG), and nonsibling related transplant was given to 2 patients each in pre-IMG and IMG. Total body irradiation-based conditioning was mostly done in pre-IMG (13/15), while busulfan-based conditioning was more common in the IMG (9/10).
OS after HSCT was 73.3% and 66.7% for pre-IMG and IMG, respectively (P=0.868), while OS for transplant recipients in first CP (CP1) was 78.6% and 85.7% (P=0.646) (Fig. 3). The OS of MSD-HSCT was higher than alternative donor (AD) HSCT (91.7% vs. 50.0%, P=0.019), and transplant in CP1 resulted in much higher OS than transplant in CP2 (81.0% vs. 0.0%, P=0.004).
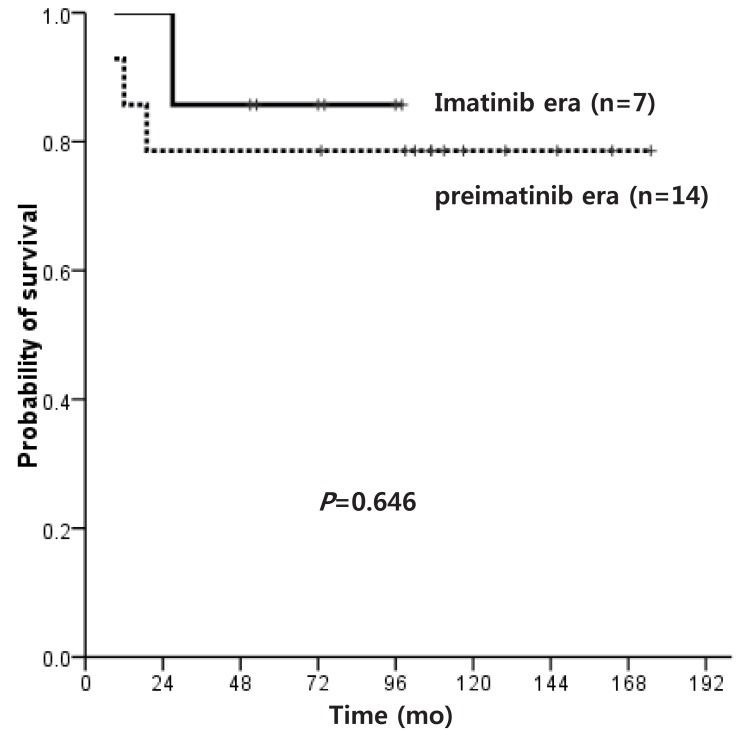
Overall survival of patients who underwent hematopoietic stem cell transplantation in first chronic phase in the preimatinib era vs. imatinib era.
PFS was 58.7% and 41.7% (P=0.149) for MSD and AD-HSCT, respectively, and 53.3% and 42.9% (P=0.912) for pre-IMG and IMG. Of the 25 transplant recipients, 7 patients died of transplant-related causes (including 4 due to chronic graft-versus-host disease), and 1 patient with autologous recovery is currently maintaining MMR while on IM. Of the 4 patients who relapsed post-transplant (2 pre-IMG, 2 IMG), 2 patients each are in CMR and MMR with IM.
2) Treatment without HSCT
The 12 patients in pre-IMG who did not undergo transplant all received hydroxyurea, supplemented by IFN-α (n=4), etoposide, cytarabine or busulfan. Of these patients, 10 died of disease progression. One patient progressed to AP, achieved CCyR with 6 months of IM, and switched to dasatinib due to skin rash. The other patient maintained CHR during 2 years of hydroxyurea, then switched to IM and is currently in CCyR.
Of the 15 patients in IMG who did not receive transplant, 2 were in advanced disease states, including 1 patient diagnosed in BC who started IM but died without response 8 months afterwards, and 1 patient diagnosed in AP who achieved CCyR in 6 months, MMR in 21 months, and is currently receiving treatment 36 months onwards. The remaining 13 patients were diagnosed in CP, and except for 1 patient, treatment response evaluation was possible in all patients. One patient started IM, but began alternative medicine treatment before achieving CHR. Of the 12 patients, 2 showed progression, including 1 patient who converted to BC and died 7 months into IM treatment, and another patient who relapsed from CHR, had a T315I mutation confirmed, began treatment with a second generation TKI, but has yet to achieve MMR.
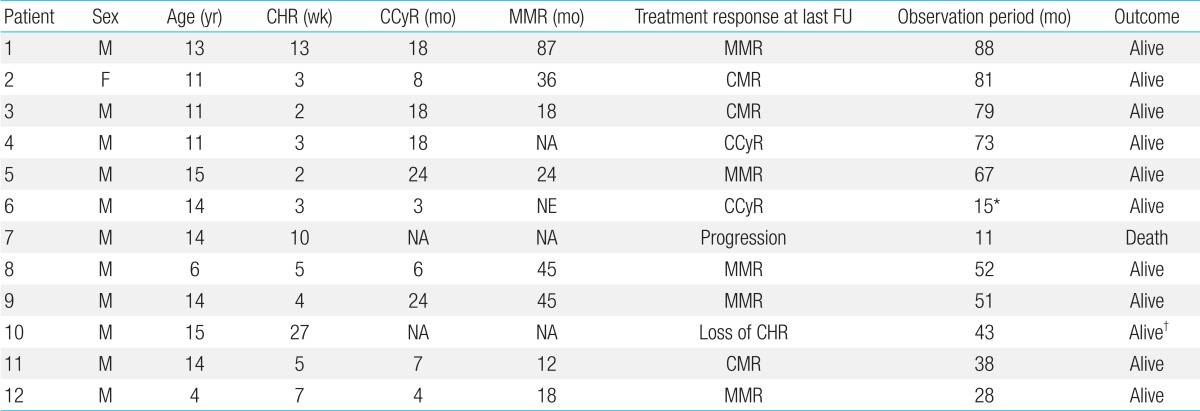
The median follow-up duration of the 12 patients who were diagnosed in CP and received continuous IM treatment was 60 months (range, 28 to 88 months, excluding one patient who died). All achieved CHR at a median of 4.5 weeks (range, 2 to 27 weeks) since treatment. Ten patients (83.3%) achieved CCyR (median, 13 months; range, 3 to 24 months), 8 (66.7%) achieved MMR (median, 35 months; range, 12 to 87 months), and 3 are currently maintaining CMR (Table 3).
Of the patients who did not receive transplant, the OS of pre-IMG and IMG was 16.7% and 85.7%, respectively (P=0.032), and the OS of patients diagnosed in CP only was 16.7% and 91.7% (P=0.014), and the 5-year PFS was 50.0% and 82.5% (P=0.035) (Fig. 4), respectively, indicating a significant survival advantage for the IMG.
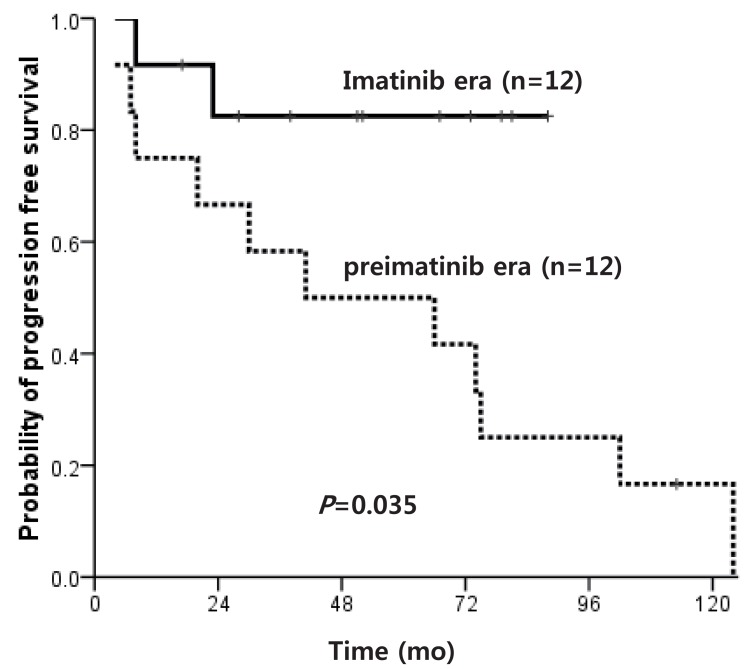
Progression free survival of patients who did not receive hematopoietic stem cell transplantation according to imatinib use.
Of patients diagnosed with CP within the IMG, the OS of matched donor HSCT recipients and IM only treated patients was similar at 83.3% and 91.7%, respectively (P=0.560). The PFS also did not show a significant difference at 54.3% and 82.5%, respectively (P=0.208) (Fig. 5), but the IM only treated patients showed a high survival plateau at 2 years follow-up.
3. Adverse effects of IM
Overall, 36 patients showed adverse effects of IM, including BM suppression, which was most frequently observed, bone or joint pain, nausea, vomiting, diarrhea, and abdominal pain (Table 4). Except for 1 patient who experienced bone pain that led to temporary withdrawal of IM, and another who changed to dasatinib due to skin rash, all adverse effects were transient and resolved with supportive care.
Discussion
Previous to the introduction of TKI's, hydroxyurea, busulfan, IFN-α, and cytarabine were used in the treatment of childhood CML, and allogeneic HSCT was regarded as the only curative mode of treatment. As in other diseases requiring HSCT, MSD transplant is known to result in better survival than unrelated transplant. Our study showed PFS of 58.7% and 41.7% for 13 MSD and 12 alternative transplants respectively, indicating better survival for sibling transplant recipients, although the numbers were too few to show statistical significance. However, past studies done on larger patient cohorts have shown considerably better leukemia-free survival in MSD transplant recipients compared with unrelated donor transplant recipients17,18). Improved outcome has been recognized when HSCT is done in CP1, and our results show that all 4 patients who received HSCT in CP2 died, except for 1 patient who has survived event-free for 10 months since transplant.
IM is the first molecularly targeted drug for CML, with efficacy proven in adults. The few studies that have reported on IM as first-line treatment in children are based on relatively small numbers of patients, and remain inconclusive as to treatment guidelines, mostly due to concern over long-term side effects9-11,19).
Millot et al.10) reported on 44 patients under the age of 17 who showed 98% PFS with a median follow-up of 31 months, while 2 other studies reported a promising 100% PFS9,19). All of these studies indicate that IM is potentially as effective as first-line treatment in children as it is in adults. The PFS of patients diagnosed in CP and treated with IM only in our study was 82.5%, which is lower than figures reported by other studies, but higher than the 58.7% PFS of MSD transplant recipients. However, considering the poorer outcome of CP2 transplant recipients, patients who progress while on IM are predicted to have low OS, especially considering that second generation TKIs are not widely available for children. Hence, an important issue that remains unresolved is the duration of IM treatment necessary for cure.
In adults, the overall treatment plan is based on cytogenetic and MRs to TKI treatment15,20), and similar treatment guidelines can be drawn for children. However, for those who do not show optimal response to IM, the subsequent treatment options are limited, especially in the pediatric setting. In our study, treatment response, graded according to a recent report21), showed CHR in 83.3% (n=10) at 3 months, CCyR in 41.7% (n=5) at 18 months, and MMR in 25% (n=3) at 18 months for patients given IM in CP1, indicating that the majority of patients should be considered for alternative treatment options. However, except for the 2 patients who progressed within 10 months, all patients continued with IM and maintained at least CCyR response. Past studies, including the French national phase IV trial10) and the study by Muramatsu et al.19) which reported 61% and 54.6% CCyR at 12 months respectively, have shown a higher CCyR rate than in our study. The MMR rate in children followed for at least 36 months has been reported to be 36.4-57%, similar to the 41.7% of our study9,10,19). Adequate serum drug levels are important for CCyR, MMR and event-free survival, emphasizing the importance of drug compliance and therapeutic drug monitoring22-24).
In this single center study, we compared the OS before and after the introduction of IM, and found a higher OS after the start of IM treatment (78.4% vs. 45.7%), although the small numbers in the cohort did not result in statistical significance. However, when considering only patients in CP, a major difference was noted in OS in favor of IMG (89.7% vs. 45.7%, P=0.025). The difference in OS held, even when patients who received transplant were included.
Efficacy of IM in patients who relapsed after HSCT has been reported previously25,26), and in our cohort, 4 patients who relapsed after transplant are alive in CMR (n=2) or MMR (n=2), reaffirming the role of IM for post-transplant treatment.
The Stop Imatinib (STIM) trial6) has introduced the option of stopping IM treatment altogether in patients who continue to maintain CMR. Such studies, however, have not been undertaken in children and long-term use of the drug raises concerns about toxicities. Various adverse events were found in our patients, although most were of mild to moderate severity. Three patients showed resolution of side effects after temporarily stopping the drug, and only 1 patient required change to another TKI. Further follow-up to detect major complications such as growth retardation is necessary27,28).
The 24- to 36-month follow-up data for dasatinib (DASISON study) and nilotinib (ENESTnd study) demonstrate superior cytogenetic and molecular response rates at certain time points and lower rates of progression to accelerated or blast phase compared to imatinib7,8). Earlier introduction of these drugs to children, who potentially require a longer period of treatment than adults, may allow for limited toxicities while optimizing efficacy.
In conclusion, for patients diagnosed in CP, first-line IM treatment resulted in higher PFS than during the pre-IM treatment period, allowing patients to avoid major consequences of HSCT, such as transplant-related mortality. Careful observation of long-term, drug-related complications should be undertaken in a multicenter setting, and clinical trials of more recently developed TKIs should be initiated in the pediatric setting.
Notes
No potential conflict of interest relevant to this article was reported.