An adverse event following 2009 H1N1 influenza vaccination: a case of acute disseminated encephalomyelitis
Article information
Abstract
Acute disseminated encephalomyelitis (ADEM) is an inflammatory demyelinating disease of the central nervous system that typically follows an infection or vaccination and has a favorable long-term prognosis. We describe the first reported case of ADEM after vaccination against novel influenza A (H1N1). A previously healthy 34-month-old boy who developed ADEM presented with a seizure and left-sided weakness 5 days after vaccination against novel influenza A (H1N1). Cerebrospinal fluid examination revealed elevated cell counts. T2-weighted images and fluid-attenuated inversion recovery images revealed multiple patchy hyperintense lesions in the frontal and parietal subcortical white matter and the left thalamus. After the administration of intravenous corticosteroid, the patient's clinical symptoms improved and he recovered completely without neurologic sequelae.
Introduction
Acute disseminated encephalomyelitis (ADEM) is an immune-mediated, demyelinating disease of the central nervous system. In 50 to 75% of all cases, the clinical onset of disease is preceded by viral or bacterial infections, mostly nonspecific upper respiratory tract infections. In children who are diagnosed with ADEM, a history of febrile event can be established in 50 to 75% of all cases1,2). Vaccine-associated ADEM is frequently observed after measles, mumps or rubella vaccination1). To date, there are no reported large population studies or estimated incidence rates reporting an association between influenza vaccination and ADEM3). We report an uncommon case involving a previously healthy 34-month-old boy in whom ADEM developed following vaccination against novel influenza A (H1N1).
Case report
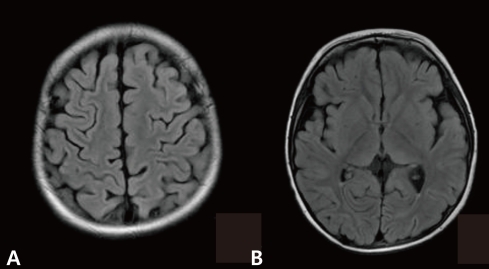
A previously healthy 34-month-old boy visited emergency room on December 2009 with a clonic seizure affecting his left hand. The seizure lasted for 90 minutes. He had no fever. He had no recent history of febrile illness and infections. He was administered a vaccination against novel influenza A (H1N1) five days ago and had no history of fever following this. He had received prior vaccination against 2008 influenza, Japanese B encephalitis, diphtheria-pertussis-tetanus (DPT), poliomyelitis, measles and hepatitis B, without complications. On assessment, his temperature was 36.6℃. He was alert and responsive to verbal commands. Muscle tone was decreased only in the left arm with grade 1 muscle power. He was able to walk limping on his left leg. Cranial nerve and sensory examination, deep tendon reflex and pathologic reflex did not show any abnormalities or pathologic findings. Real-time reverse transcription-polymerase chain reaction (RT-PCR) for H1N1 influenza was negative. Spinal tap revealed clear and colorless cerebrospinal fluid (CSF) containing 58 white cells/µL (100% lymphocytes). CSF protein level was 32.1 mg/dL and glucose level was 57 mg/dL (serum glucose concentration 93 mg/dL). Gram staining and culture were negative. In the CSF, PCR for enterovirus and herpes simplex virus were negative. Tests for Hemophilus influenzae, Streptococcus pneumoniae and Neisseria meningitidis antigens were negative. Complete blood count, erythrocyte sedimentation rate, C-reactive protein, blood culture, liver and renal function test and sleep electroencephalogram were normal. Magnetic resonance imaging (MRI) revealed multiple patchy hyperintense lesions in the frontal and parietal subcortical white matter (Fig. 1A) and the left thalamus (Fig. 1B). Based on the diagnosis of ADEM, 1 mg/kg of dexamethasone was administered to the patient intravenously for five consecutive days. On the third day of treatment, the patient's left arm had returned to near normal. On the fifth day, he was able to walk without limping. On the sixth day, he was able to walk normally without weakness on the left side and was discharged. One month later, follow-up MRI showed resolution of the previous lesions (Fig. 2). To date, twelve months later, he has no neurologic deficits.

Fluid-attenuated inversion recovery images (TR/TE/TI=11000/105/2600 ms) at initial presentation demonstrate multiple patchy hyperintense lesions in the subcortical white matter (A) and the left thalamus (B).
Discussion
ADEM is thought to be a neurologic complication following infection or vaccination, caused mainly by a T cell-mediated immune reaction to myelin components or oligodendrocytes4). ADEM may occur as a complication of vaccinations including Japanese B encephalitis vaccine, live rubella vaccine, hepatitis B vaccine, diphtheria-tetanus-pertussis and influenza vaccine. For most vaccines, the incidence rates are as low as 0.1 to 0.2 per 100,000 vaccinated individuals.
The association between the influenza vaccination and ADEM has only come to light in recent years, and hence there have been no large population studies or estimated incidence rates3). Of the total 460 adverse events associated with intranasal influenza vaccine between 2003 and 2005 in the USA, 10 neurological events were reported that included two people with Guillain-Barre syndrome (GBS), one ADEM, and one febrile convulsion. Nakayama and Onoda found three cases of ADEM and nine of GBS among those who received 38.02 million doses of influenza vaccine administered between 1994 and 2004 by the Kitasato Institute, Japan3). During the 2001 to 2002 influenza season, 9,842,601 doses of influenza vaccine were distributed to health care providers in Canada, although the exact number of doses administered is unknown. Health Canada received 1,800 reports of people experiencing adverse events following the influenza vaccine, representing a rate of 183 reports per one million doses distributed. Of these, the serious adverse events reported were GBS, convulsions, and meningitis and encephalopathy (1 per 3 million doses distributed)5).
In the case presented here, there was no evidence of a febrile illness or infection in our patient before or after vaccination against novel influenza A (H1N1), and ADEM developed five days after vaccination. Although it is difficult to establish a causal relationship between ADEM and vaccination, the close temporal association of the influenza vaccination with the neurologic symptoms further suggests a possible association with the vaccine6,7). The patient was treated with intravenous corticosteroid as first line therapy8), which produced a complete recovery without neurologic sequelae. Follow up MRI findings showed an improved state and no new lesion.
From October 2009 to June 2010, Koreans have received 14,762,293 doses of the vaccine against novel influenza A (H1N1) according to vaccine adverse events reporting system (VAERS)9). The Korea Centers for Disease Control and Prevention reported 88 cases of people who were diagnosed with adverse events following this vaccination. Of these, the serious adverse events included GBS in ten people (two per three million vaccinated individuals), ADEM in two (0.4 per three million vaccinated individuals) and Miller Fisher syndrome, convulsion, cerebellitis and meningitis each in one case (Adverse events of novel influenza A [H1N1] 2009 vaccination in South Korea: VAERS, unpublished data).
In summary, post-vaccination ADEM has been already reported associated with several vaccines. However, there has been no previous report of ADEM associated with mass vaccination against novel influenza A (H1N1). Although causal relationship cannot be established, we report here a rare adverse event, a case of ADEM following vaccination against novel influenza A (H1N1).