Transient asymptomatic white matter lesions following Epstein-Barr virus encephalitis
Article information
Abstract
We present the case of a patient with Epstein-Barr virus (EBV) encephalitis who developed abnormal white matter lesions during the chronic phases of the infection. A 2-year-old-boy was admitted for a 2 day history of decreased activity with ataxic gait. The results of the physical examination were unremarkable except for generalized lethargy and enlarged tonsils with exudates. Brain magnetic resonance imaging (MRI) at admission showed multiple high signal intensities in both basal ganglia and thalami. The result of EBV polymerase chain reaction (PCR) of the cerebral spinal fluid was positive, and a serological test showed acute EBV infection. The patient was diagnosed with EBV encephalitis and recovered fully without any residual neurologic complications. Subsequently, follow-up MRI at 5 weeks revealed extensive periventricular white matter lesions. Since the patient remained clinically stable and asymptomatic during the follow-up period, no additional studies were performed and no additional treatments were provided. At the 1-year follow-up, cranial MRI showed complete disappearance of the abnormal high signal intensities previously seen in the white matter. The patient continued to remain healthy with no focal neurologic deficits on examination. This is the first case of asymptomatic self-limited white matter lesions seen in serial MRI studies in a Korean boy with EBV encephalitis.
Introduction
Epstein-Barr virus (EBV) is a member of the herpesvirus family. EBV infections have diverse clinical manifestations including subclinical infection, infectious mononucleosis (IM), and associations with malignancies such as nasopharyngeal carcinoma, Burkitt lymphoma, Hodgkin's disease, and primary central nervous system lymphoma. Numerous neurologic manifestations associated with EBV have been documented, including encephalitis, aseptic meningitis, transverse myelitis, Guillain.Barre syndrome, cranial nerve palsies, and seizures occurring alone or in the setting of infectious mononucleosis1,2). Neuroimaging studies of EBV encephalitis are normal in most patients, but there have been some reports of increased signal intensities in both basal ganglia, thalami, and brainstem area during the acute stages of the illness1-3). However, little data regarding the neuroradiologic findings associated with subacute or chronic stages of EBV encephalitis have been reported in the medical literature4,5). There have been several case reports of abnormal white matter lesions on successive brain magnetic resonance imaging (MRI) following herpes simplex encephalitis (HSE) regardless of clinical symptoms6-11). But there has been no report demonstrating increased white matter signal intensities on serial MRI following EBV encephalitis without clinical manifestations. We present a case of a 2-year-old-boy demonstrating asymptomatic white matter lesions during the chronic phase after EBV encephalitis.
Case report
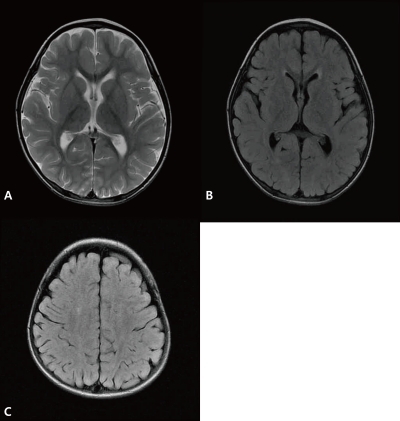
A 2-year-old-boy was admitted to our hospital with a 2-day history of decreased activity with gait disturbance. He had an 11-day history of coughing, rhinorrhea and 8-day history of fever prior to admission. He had no significant past medical history. On admission, he was lethargic and his body temperature was 36.9℃. Physical examination showed enlarged tonsils bilaterally with exudates. Neurologic examination was unremarkable except for an unsteady gait. Laboratory findings included a leukocyte count of 15,200/µL (segmented neutrophil 28.1%, lymphocyte 58.8%, and monocyte 11.5%), hemoglobin 11.3 g/dL, platelet count 439,000/µL, serum sodium 139 mEq/L, potassium 4.4 mEq/L, chlorine 99 mEq/L, blood urea nitrogen 8.0 mg/dL, creatinine 0.6 mg/dL, glucose 91 mg/dL, ammonia 34 µmol/L, and plasma lactate/pyruvic acid 2.44/0.55 mmol/L. Cerebrospinal fluid (CSF) analysis was contaminated with blood due to a traumatic tap. CSF analysis revealed red blood cell 330,000/µL, white blood cell 100/µL (lymphocyte 75%), protein >300 mg/dL, glucose 71 mg/dL, CSF immunoglobulin (Ig) G index 1.12 (<0.85), with a negative results for oligoclonal band. Brain MRI revealed multiple high signal intensities in both basal ganglia and thalami (Fig. 1). CSF for EBV polymerase chain reaction (PCR) was positive and serologic testing was compatible with an acute EBV infection: positive for viral capsid antigen (VCA)-IgM, negative for VCA-IgG, EBV early antigen (EA)-IgG, and EBV nuclear antigen (EBNA)-IgG. CSF PCR for herpes simplex type I, II, Mycoplasma pneumoniae, and Mycobacterium tuberculosis were all negative. CSF viral cultures for influenza type A, B, and enteroviruses were negative. No bacterial organism was isolated from patient's blood, stool, and urine. An electroencephalogram showed abnormal localized slowing over the left hemisphere and right posterior head. Encephalitis was diagnosed by clinical and radiologic findings, and empirical intravenous antibiotics and acyclovir were administered, but stopped after the final negative CSF results for microorganisms were obtained. He recovered gradually without neurologic deficits and was discharged after the 7th day of admission. At 5 weeks after the onset of illness, he revealed no neurological symptoms or deficits on examination. However, follow-up brain MRI demonstrated new multiple high signal intensities in both periventricular white matter with interval disappearance of previous lesions on T2-weighted and fluid attenuated inversion recovery (FLAIR) images (Fig. 2). Since he remained clinically silent during the follow-up period, no additional studies were performed and no treatments were initiated. Serologic testing for EBV was compatible with recent past infection: positive for VCA-IgG, negative for VCA-IgM, EA-IgG, and EBNA-IgG. Brain MRI performed at 1 year after onset of the disease showed complete disappearance of previous white matter abnormal signal intensities with no newly developed lesions (Fig. 3). During the 1-year follow-up, the patient continued to remain healthy with no neurological deficits.
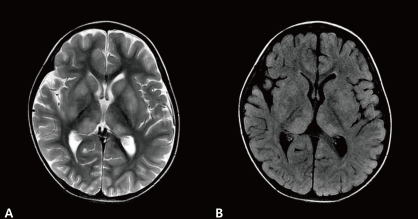
T2-weighted image (A) and fluid attenuated inversion recovery (B) brain magnetic resonance imaging showed increased high signal intensities in both basal ganglia and thalami with intact white matter on admission.
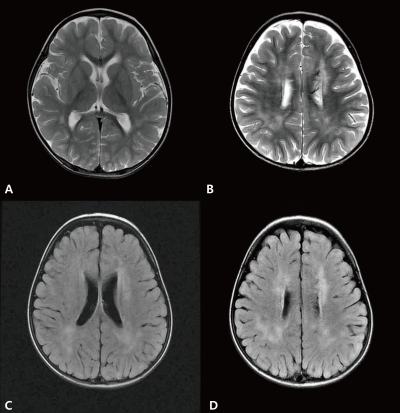
T2-weighted magnetic resonance (MR) image demonstrated disappearance of previous signal changes in both basal ganglia and thalami (A). T2-weighted MR image (B) and fluid attenuated inversion recovery magnetic resonance imaging (C, D) revealed newly developed extensive periventricular white matter high signal intensities in both hemispheres at 5 weeks after disease onset.
Discussion
EBV is a well-known pathogen for IM and a herpesviridae family member. EBV infections have various neurologic manifestations alone or accompanied by clinical features of IM1-3). According to one study on pediatric EBV associated encephalitis, EBV infection was demonstrated in 9.7% of children admitted with acute encephalitis. EBV is one of the more common identifiable causes of acute childhood encephalitis and remains the most common agent mimicking HSE2). The prognosis associated with EBV encephalitis is controversial. Some reports have suggested it to be a relatively benign, self-limited disease with almost full recovery. Others have documented the occurrence of various neurologic sequelae in a substantial number of cases2,3). In one pediatric study, 40% had residual neurologic sequelae that included global impairment, autistic-like behavior, and focal paresis3). Most children have a benign clinical course without neurologic sequelae, but 10% have residual persistent deficits and a mortality rate of up to 10% has been reported1,2). Since EBV has a tropism for the deep nuclei, neuroimaging can show characteristic multiple foci of T2-weighted or FLAIR hyperintensity in the hemispheric cortex, brainstem, bilateral thalami, basal ganglia, and/or splenium1-3,12,13). Rarely, extensive white matter lesions have been reported in patients with acute disseminated encephalomyelitis (ADEM) associated with chronic EBV infection and clinical relapse of neurologic problems4,5). However, there have been no reports documenting sequential cranial MRI findings in asymptomatic patients following EBV encephalitis. Our patient suffered from EBV encephalitis coincidentally with IM and recovered completely without neurologic sequelae. He was followed up at our outpatient clinic regularly for close observation of neurologic complications. The follow-up brain MRI was performed for the purpose of demonstrating resolution of initial deep nuclei lesions seen on presentation. However, his follow-up MRI revealed extensive periventricular white matter hyperintensities resembling that of an ADEM patient. Despite his multiple cerebral lesions on brain MRI, the patient remained clinically stable with no focal neurologic deficits. Subsequently, white matter lesions completely disappeared on the third brain MRI one year later. Although our patient could be diagnosed as having ADEM following EBV encephalitis according to the neuroradiologic features of his second brain MRI, he did not display any evidence of encephalopathy or clinical features compatible with ADEM at that time. As a result, his neuroradiologic findings are believed to represent one spectrum of post infectious autoimmune-mediated demyelinating lesions. A secondary immune-mediated process has been suspected in some patients with transient or chronic white matter involvement occurring after HSE. These patients were asymptomatic or developed an acute or chronic secondary neurologic deterioration without extrapyramidal movement disorder6-11,14). Though the exact mechanisms responsible for the transient white matter lesions observed after HSE remain unclear, possible explanations include a postinfectious immune-mediated demyelination process or prolonged edema or gliosis induced by chronic stimulation of the immune system in response to viral infection6-11,14). In addition, white matter lesions have also been noted to be related to intravenous acyclovir in some cases15). This is the first report of a patient with transient diffuse white matter hyperintensities following EBV encephalitis in the absence of neurologic symptoms or signs. EBV is a family member of herpersviridae and is known to be the most common virus mimicking HSE in clinically. As a result, the white matter lesions following EBV infection could also resemble that of HSE. Given that we did not pursue further studies after finding his new white matter lesions, potential limitations in accurately diagnosing this patient remains. Since this was the first case report demonstrating a patient with asymptomatic self-limited white matter lesions during the chronic phase following EBV encephalitis, more clinical data and future research is needed to better characterize and determine the significance of our findings.