Usefulness of drug provocation tests in children with a history of adverse drug reaction
Article information
Abstract
Purpose
There are very few reports of adverse drug reactions (ADR) and almost no study of drug provocation test (DPT) in Korean children. We aimed to assess the role of DPT in children with unpredictable ADRs, and compare the causative drugs and clinical characteristics between detailed history of ADRs and result of DPTs.
Methods
We included 16 children who were experienced ADRs referred to pediatric allergy clinic at Ajou University Hospital (January 2006 to December 2009). With various suspected drugs, 71 DPTs were done in 16 patients using our own protocol, and skin tests to antibiotics were combined in ADRs to antibiotics in medical history.
Results
There were 17 (23.9%) positive DPTs results out of 71 individual DPTs, and 11 patients (68.8%) from 16 patients were positive to at least one drug. Drugs causing positive reactions were acetaminophen in 5 (31%), Non-steroidal anti-inflammatory drugs in 4 (25%), penicillin in 3 (19%), cephalosporin in 2 (13%), and cotrimoxazole, macrolide and lactose in 1 each.
Conclusion
DPT seems a safe and useful procedure to confirm causative drug and identify safely administering alternative drugs in children with ADR.
Introduction
Drugs which are used to prevent, treat and research diseases sometimes cause unwanted reactions, so called adverse drug reactions (ADRs) and the World Health Organization (WHO) has defined ADR as an unplanned and unintended harmful effect of a drug despite its being administered at a an adequate dose1,2). By a systemic review and meta-analysis of prospective studies, ADR has been reported to vary from 5 to 17%. The overall incidence was 10% in hospitalized children and 2% in outpatient children, and the overall rate of pediatric hospital admissions due to ADRs was 2%1). In Korea, there is a national wide survey for prevalence of asthma and other allergic diseases by internationally validated questionnaire. By this survey, last twelve-month prevalence of drug hypersensitivity was 2% in 40,429 school children aged 6 to 15 years3), but there has been almost no report of clinical studies about ADRs in children, except several case report about fixed drug eruption in children4-6).
Majority of ADRs are predictable (type A) and 20% of ADRs are unpredictable (type B) which are unrelated to the dose and pharmacological mechanism of individual drugs. Many of the symptoms of type B ADRs require emergency treatments, furthermore, unpredictable ADRs are especially problematic in pediatric patients1,2,7). ADRs are common in children and can be life-threatening and but the false positive diagnosis are prevalent in clinical settings, especially in the children with fever or infections1,2,8,9). So the confirmation of the diagnosis should be performed by detailed clinical history and a physical examination, possibly followed by drug provocation tests (DPT)7,10).
In this study we aimed to assess the role of DPT in children with unpredictable ADRs, and compare the causative drugs and clinical characteristics between detailed history of ADRs and result of DPTs.
Materials and methods
1. Patients
We include 16 patients out of 32 patients under 18 years of age, who, over 4-year period (January 2006 to December 2009), were referred to the pediatric allergy department at Ajou University Hospital, due to complete past history of ADRs. Medical records were reviewed retrospectively, demographic data and detailed clinical history such as experienced allergic reactions after ingestion of drugs, severity of ADRs, and combined disease, and result of DPT with suspected drugs. ADR was considered mild if the drug was stopped or switched without any particular treatment, and severe if the patient had to be examined at a tertiary medical center or hospitalized for treatment11).
All 16 patients have one of the clinical symptoms of urticaria, maculopapular eruption, isolated generalized pruritus, laryngeal edema, bronchospasm, rhinoconjunctivitis, or anaphylaxis. We excluded patient with nonimmediate chronology (8 hours after the last drug administration) (n=8), symptoms disappearing without cessation of the suspected drug. We also excluded the patients with chronic urticaria (n=2), food allergy, who had experienced severe life-threatening skin reaction (including vasculitis, exfolative dermatitis, toxic epidermal necrolysis or Stevens-Johnson syndrome), and parents' opposition about DPT (n=6).
2. Skin test and drug provocation test
After receiving informed consent from parents, we performed DPT in 16 patients who showed evidence of ADRs, and we followed general guidelines for DPT with the modification for skin test before DPT, dose and time interval during DPT12). DPT was performed in good health, and at least 4 weeks after disappeared ADRs in each patient. All DPTs were performed on an inpatient basis. The blood vessel was secured, and epinephrine and a short-acting steroid were prepared in advance against emergencies such as anaphylaxis and shock. During each test, physicians present to observe clinical symptoms and measure vital signs every 15 minutes for the first one hour, every 30 minutes for the next two hours, and every hour for the next three hours. In patients who were suspected to have symptoms after administration of an oral antibiotic, an intradermal skin test was done before DPT. Skin test was performed using intravenous antibiotic of the same element. For the skin test we use the 1:1,000 diluted antibiotic solutions and followed the usual method. The amount of induration or redness in response to the test measured by trained person 15 minutes after administration. When skin test reactivity in present, it causes >10 mm of induration.
For the DPT, we used commercial preparation of suspected drug. We use the 25% of a usual therapeutic dose as a starting dose, 50% as a 2nd dose, and 100% as a 3rd dose, and the interval was 30 to 60 minutes. If there was symptom provoked, the test were stopped and interpreted as positive. If there was no symptom during this first course of DPT, a 100% of therapeutic dose of drug was administered 4 to 6 hours after the 3rd dose of test, and the symptom were observed for at least 8 hours after the last dose of test. Then the other kinds of drugs were tested to diagnose or offer a alternative drugs with the same method.
Results
1. Profile and clinical characteristics of patients by history
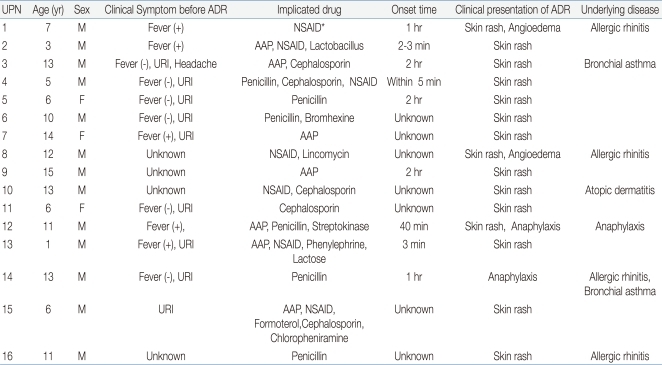
Thirteen (81%) out of 16 patients were males and 3 (19%) were females, making the male-female ratio 4.3:1. Median patient age are 9.1 years (range, 1 to 15 years), with 3 patients (19%) younger than 6 years, and 13 (81%) 6 years or older. The most frequent clinical pattern of ADR observed in these patients was skin rash, which occurred in 15 children (93%) followed by angioedema and anaphylaxis in 2 each (13%). Five (31%) patients have allergic rhinitis, 2 (13%) have bronchial asthma and 1 has atopic dermatitis as underlying diseases (Tables 1, 2). And 4 patients experienced angioedema or anaphylaxis as ADRs had underlying allergic disease such as allergic rhinitis or bronchial asthma (Table 2). As to severity, 3 (16%) patients showed severe ADR.
Twelve out of 16 patients took the drug due to febrile illness or upper respiratory illness, before or during experienced ADRs. The most common drug suspected of causing ADRs, obtained from patient inquiry and assessment of medical records, was antibiotics in 13 patients (81%), including 6 (38%) with penicillin antibiotics, 5 (31%) with cephalosporin antibiotics, and 2 with (13%) macrolide antibiotics. Non-steroidal anti-inflammatory drugs (NSAIDs) were suspected in 7 children (44%), acetaminophen in 7 (44%), and enzyme (lactose) in 1 (Table 2). The onset time of ADRs was several minutes in 3 patients, less than 2 hours in 6 patients, and unknown in 7 patients.
2. Drug provocation test
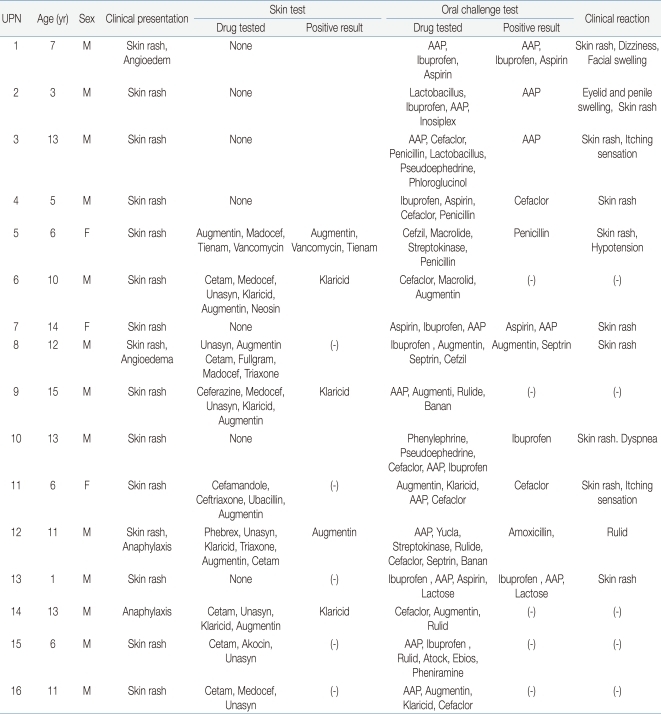
The results of DPTs and skin tests were shown in Table 3. In 16 patients, 71 DPTs were performed using various drugs for confirm the causes of ADRs or finding alternative drugs. There were 17 (23.9%) positive DPTs results out of 71 individual DPTs. And 11 patients (68.75%) were positive to at least one drug. Drugs causing positive reactions were acetaminophen in 5 (31%), NSAIDs in 4 (25%), penicillin in 3 (19%), cephalosporin in 2 (13%), and cotrimoxazole, macrolide and lactose in 1 (6%) each (Fig. 1). When we assessed the correlation between the drug suspected of causing ADR based on clinical history and the result of DPT, we found that 4 of 8 patients with DPTs (50%) showed positive reactions to NSAIDs. In addition, 5 of 11 (45%) showed positive reactions to acetaminophen, 3 of 10 (30%) to penicillin antibiotics, 2 of 11 (18%) to cephalosporin antibiotics, 1 of 8 (12%) to macrolide antibiotics, 1 of 2 (50%) to cotrimoxazole and 1 of 1 (100%) to lactose.
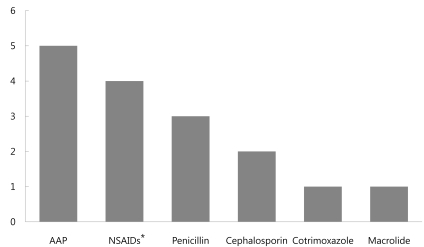
Drugs causing positive reactions were acetaminophen in 5 (31%), non-steroidal antiinflammatory drugs (NSAIDs) in 4 (25%), penicillin in 3 (19%), cephalosporin in 2 (13%), and cotrimoxazole, macrolide and lactose in 1 (6%) each. AAP, acetaminophen. *NSAIDs include aspirin and ibuprofen in this study.
Among 16 patients, 8 (50%) had skin tests before the DPT test in order to estimate the putative severity of ADR for treatment with antibiotics. Three patients (patient 5, 6, and 12) showed positive reactions to the same drug in both the skin and drug provocation tests, whereas 2 patients (patient 8 and 11) were negative on the skin reaction tests but positive on the DPTs.
Clinical symptoms indicating positive DPTs included skin rash in 11 of 16 (68%) patients, angioedema in 2 (18%), and pruritus in 2 (18%). In addition, dizziness, anaphylaxis, dyspnea, and abdominal pain accompanied by vomiting occurred in 1 each. In 4 patient who had angioedema or anaphylaxis by previous history of ADRs, only one DPT reproduced the similar symptoms and albeit milder. DPTs provoked dyspnea or hypotension in 2 patients who had only skin rashes by history of ADRs. In 7 patients with underlying allergic diseases, 5 (71.4%) patients were positive in DPTs. In 5 patients with ADRs were related to fever, all patients reproduced positive DPTs, and in 10 patients with ADRs related with upper respiratory infection symptom (URI), DPTs positive in 7 (70%) patients.
Discussion
The major result of this study is that positive DPT result occurred in 11 (69%) patients in 16 patients with a history of ADRs. According to the individual DPTs, there were 17 (23.9%) positive DPTs in 71 events of DPTs. In this study the positive rate of DPTs are relatively higher than those of other studies7,13). In one retrospective study performed in children and adult ADRs, they confirmed that 241 (17.6%) of 1,372 patients were positive on the drug provocation test7).
In our study, we found that the majority of ADRs were due to antipyretics or NSAIDs which were used to manage febrile illness for control of proportion of positive test results was higher for NSAIDs (50%) and acetaminophen (45%) than for penicillin antibiotics (30%), cephalosporin antibiotics (18%) and macrolide antibiotics (12%). This result is similar to previous study that aspirin (47.2%), other NSAIDs (27.3%) than for macrolides (13.7%), and β-lactams (8.4%)7). A negative drug provocation test result is important to the patient with suspected drug allergy because reduce the meaningless avoidance suspicious drugs in the future. The DPT result may have been falsely negative in some patients with mild sensitivity or a long delay between drug hypersensitivity reaction and allergy evaluation. For these reason we require more careful attention to analyzed DPT in many cases. Especially in children, there is a tendency that mild ADRs are under documented in the patients' record, while there are over diagnosis of ADRs because of the general occurrence of rashes are common during a course of febrile illness in children7,9,11). However, in our study, febrile illness or URI did not cause false positive history of ADRs in patients, because 100% and 70% of patients with fever or URI showed positive reactions by DPTs which performed disease free or fever free conditions in all patients. While DPT is still the gold standard for identification of a causative drugs, a complete work-up is required to diagnose drug hypersensitivity: a detailed clinical history and physical examination, followed by 1 or more skin tests combine with DPT, measurement of tryptase of histamine in some cases10-15). Because of the discrepancy between ADR history and result from DPT, it is important to perform DPT for correct diagnosis and avoiding over diagnosis of ADRs, even though every ADR patients cannot be performed DPTs for diagnosis.
Drug hypersensitivities may be manifested by various symptoms in different organs, with skin reaction being the most common2,16). We found that 15 of 16 (93%) of our patients with ADR presented with skin lesions in detailed medical history before drug provocation tests. In our study, clinical symptoms indicating positive DPTs included skin rash in 11 of 16 (68%) patients, angioedema in 2 (18%), and pruritus in 2 (18%). In addition, dizziness, anaphylaxis, dyspnea, and abdominal pain accompanied by vomiting occurred in 1 each, and these results are similar patterns to other studies7,13). Because drug allergies may involve organs other than the skin and skin lesions may appear in various patterns, the presence of a skin lesion per se is not helpful in diagnosing the allergy or determining its method of treatment17).
A DPT is the controlled administration of a drug in order to diagnose drug hypersensitivity reactions and identify a alternative drug in patient with ADR to specific drug. While there are some modification of method of doses to perform DPTs, there are recommended general considerations DPTs2,10,12). In general, DPTs start with a challenge with a low dose of drug, followed by gradual increases. In our hands, DPTs were effective and safe. With careful selection of patients, the progressive administration of the drug with a small starting dose, and strict medical surveillance, no positive reaction was too severe to respond promptly to treatment.
Theoretically, skin tests for antibiotics assay for immunoglobulin E (IgE)-mediated adverse reactions18). However, in most patients, it is impossible to distinguish between IgE-mediated and non-IgE-mediated allergic reactions19). For example, among patients positive for penicillin skin reactions, 33% did not have a previous history of penicillin allergy20), and ADR after the administration of penicillin was not observed in 60.9% of patients positive on penicillin skin tests, with only a very small number of patients showing mild reactions during oral provocation tests12). In our study, three patients (patient 5, 6, and 12) showed positive reactions to the same drug in both the skin and DPTs, whereas 2 patients (patient 8 and 11) were negative on the skin reaction tests but positive on the DPTs. About 0 to 10% of such patients are resensitized; that is, they are negative on a first skin reaction test but positive on a subsequent skin reaction test21-23). These changes, however, are not relevant to the frequency of ADR in DPTs and cannot explain the discrepancy between the results of skin reaction and DPTs2). It is generally inadvisable to administer a drug to patients positive on skin tests22), but some may have been positive on a previous skin reaction test and then became negative; these patients may not experience ADRs, even after administration of the optimal dose in DPTs2,22).
Because this study was performed retrospectively based on patient medical record, patients could be omitted by the incomplete or incorrect records. The major result of this study is that positive DPT result occurred in 69% patients with a history suggesting possible drug allergy, and we identified that the exact cause of drug for ADR in patients experienced ADRs to more than one kind of drugs at the same time. And we found that proportion of positive test results was higher for NSAIDs and acetaminophen than for other antibiotics. There was discrepancy between the results of skin reaction and DPT, we need more study about drug provocation test and guidelines for diagnosis of drug allergy.
In conclusion, we performed 71 DPTs in 16 ADR patients, and confirmed causative drugs of ADRs, and identified safe drugs even though suspicious medical history of ADRs. We also confirmed DPT is a useful and safe tool for diagnose drug hypersensitivity in some patients with ADRs need to identify real causative drugs or alternative drugs.