Clinical characteristics and prevalence of vitamin D insufficiency in children less than two years of age
Article information
Abstract
Purpose
To evaluate the clinical characteristics of vitamin D deficiency and its association with iron deficiency anemia (IDA).
Methods
A total of 171 children aged less than two years underwent 25-hydroxyvitamin D3 tests between January 2007 and July 2009. The study was classified into two groups: normal and vitamin D insufficiency, by their vitamin 25-hydroxyvitamin D3 levels.
Results
In total, 120 children were in the normal group (mean age, body weight and heights 12.5±7.0, 9.3±0.9 kg and 76.8±1.1 cm), and 51 children in the vitamin D insufficiency group (9.9±5.4 months, 9.0±0.9 kg and 75.1±0.9 cm). Vitamin D insufficiency was most commonly diagnosed in the spring (44%). The proportion of complete breast-feeding was higher in the insufficiency (92%), and 25.5% of the children in the deficient group also experienced IDA compared that 12% of normal group. Ten children in the insufficiency group experienced bony changes. Six children received calcitriol medication in the normal group, in whom the mean vitamin 25-hydroxyvitamin D3 level increased from 39.6±14.6 ng/mL (pre-medication) to 41.8±17.2 ng/mL (post-medication), and 13 in the insufficiency group, in whom the mean vitamin 25-hydroxyvitamin D3 increased from 20.7±7.0 ng/mL to a mean post-treatment level of 43.7±23.8 ng/mL.
Conclusion
This study demonstrated that approximately 30% of children aged ≤2 years experienced vitamin D insufficiency associated with subclinical rickets. Many children also experienced concurrent IDA. Guidelines for vitamin D supplement in such children must therefore be established.
Introduction
Vitamin D deficiency causes rickets by bone demineralization. In the affluent twentieth century, vitamin D deficiency was thought to be infrequent, but reports on this condition have been on the increase recently1-5). Several studies encompassing large Unites States of America (USA) populations have demonstrated that infants, children and pregnant women have significantly high prevalence of vitamin D deficiency6-9). In addition, numerous studies on vitamin D deficiency have been reported in the Korean literature. Furthermore, there have been increasing number of reports on subclinical vitamin D deficiency and subclinical rickets. Our previous studies on subclinical rickets showed that children who were breast-fed without vitamin D supplement, or those admitted from outpatient clinics with infections, were frequently found to have high prevalence of this condition, which is also associated with iron deficiency anemia (IDA). As such, it is thought that there is need to screen children with vitamin D deficiency, and to administer vitamin D supplements10,11). Previously, Grindulis et al.12) have documented concurrent iron and vitamin D deficiency in Asian infants. In a recent surveillance, vitamin deficiency and insufficiency was so common in Korean mothers and their newborn infants, that 8.3% of mothers and 22.2% of newborns were vitamin D deficiency, and 70% of both mothers and newborns were insufficiency13). In breast feeding infants, cases with both of vitamin deficiency and IDA were reported. Canpro analysis performed on IDA infants showed that iron and vitamin D were less than 40% and 30% of recommended intakes, respectively14,15). The aim of this study was to evaluate the clinical characteristics of subclinical rickets in children, and its association with IDA.
Materials and methods
A total of 261 outpatient and inpatient children underwent 25-hydroryvitamin D3 tests at the Department of Pediatrics, Eulji General Hospital between January 2007 and July 2009. We excluded the children with disease such as acute and chronic hepatitis, the children have elevated C-reactive protein which can affect the level of hemoglobin and ferritin. The number of children <6 months were 19, 6 to 12 months were 80, 12 to 24 months were 72. We retrospectively analyzed these 171 children, their clinical characteristics, blood test results, wrist radiograph results and treatment outcome. A serum vitamin D concentration of ≥30 ng/dL (75 nmol/L) was defined as being normal, and that of below 30 ng/dL (75 nmol/L) was defined as vitamin D insufficiency1,15-17). Subclinical rickets indicates vitamin D deficiency without clinical symptoms such as bony changes or seizure. Although subclinical rickets is not officially recognized medical term, it was used in several recent studies. In other words, subclinical rickets only manifest as reduced serum levels of vitamin D and/or increased alkaline phosphatase (ALP), as well as minimal radiological/biochemical bony changes10,18-20).
We defined osteopenia and rickets on X-ray by reading of radiologist. The cortex thinning and trophic lines are characteristic of osteopenia, and findings such as ill-demarcated, central cup-shaped depression in the end of ulna or radius are characteristic of rickets.
In all children, serum levels of 25-hydroxy vitamin D3, hemoglobin (Hb), hematocrit (Hct), iron, ferritin, transferrin saturation, calcium, phosphate, ALP and intact parathyroid hormone (iPTH) were measured. Birth history, past medical history, and clinical information such as height and body weight were collected from the medical records. Data are expressed as the mean±SD. Statistical analyses were performed using SPSS ver.13.0 (SPSS Inc., Chicago, IL, USA). All statistical comparisons were made with the independent sample test. Factors affecting serum vitamin D concentration were examined by using Pearson's correlation coefficients and multiple regression analysis. A P value of less than 0.05 (P<0.05) was considered to be statistically significant.
Results
1. Clinical characteristics
1) Normal group
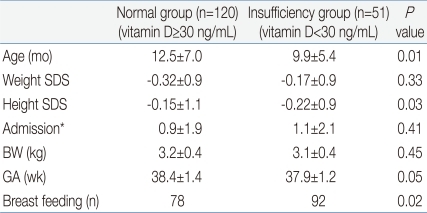
Of the 171 children enrolled, 120 (70.2%) had a vitamin D concentration of ≥30 ng/dL (normal group), and their mean age was 12.5±7.0 months. The mean body weight was 9.3 kg (standard deviation score [SDS], -0.32±0.9), and the mean height was 76.8 cm (SDS, 0.15±1.1). The mean birth weight was 3.2±0.4 kg, the mean gestational age was 38.4±1.4 weeks, and the mean annual frequency of hospitalization until the time of initial diagnosis was 0.9±1.9. Children in the normal group suffered respiratory disease most frequently (40.3%), followed by gastrointestinal disease and atopic dermatitis. Ninety-three children (78%) were breast-fed (Table 1).
2) Vitamin D insufficiency group
Of the 171 children analyzed, 51 (29.8%) had a vitamin concentration of less than 30 ng/dL (insufficiency group). The mean age was 9.9±5.4 months, which was significantly younger than that of the normal group (P<0.05). The mean height was 75.1 cm (SDS, -0.22±0.9), which was significantly lower than in that of the normal group. Although there was no significant difference in the mean hospitalization frequency between the 2 groups, the frequency tended to be higher in the insufficiency group, albeit without statistical significance. Similar to the normal group, respiratory and gastrointestinal diseases were frequently found in the insufficiency group. There were more breast-fed children in the insufficiency group than in the normal group (92% vs. 78%, P<0.05) (Table 1).
As for seasonal distribution, in the spring (March through May), vitamin D insufficiency was diagnosed in 25 of 57 (44%) children who underwent 25-hydroxyvitamin D3 tests. In summer, autumn and winter, the children of vitamin D insufficiency diagnosed respectively 9 of 41 (22%), 5 of 33 (15%), 12 of 40 (30%).
2. Blood test results
1) Normal group
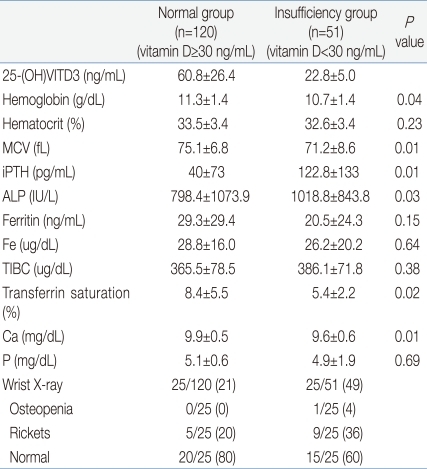
At the time of initial diagnosis, the mean 25-hydroxyvitamin D3 level was 60.8±26.4 ng/mL, the mean Hb was 11.3±1.4 g/dL. The mean serum calcium level was 9.9±0.5 mg/dL, the mean serum phosphorus level was 5.1±0.6 mg/dL, the mean serum ALP level was 798.4±1073.9 IU/L, and the mean serum iPTH level was 40±73 pg/mL. The mean serum ferritin level was 29.3±29.4 ng/mL, the mean serum iron level was 28.2±16.0 mg/dL, and the mean transferrin saturation level was 8.4±5.5%. Iron deficiency anemia was detected in 12% of children. Of the 120 children, 25 showed increased ALP levels or clinical symptoms and signs suspicious of rickets. Wrist radiographs taken in these 25 children demonstrated rickets (n=5) and normal findings (n=20) (Table 2).
2) Vitamin D insufficiency group
At the time of initial diagnosis, the mean serum 25-hyctroxyvitamin D3 level was 22.8±5.0 ng/mL. The mean serum Hb level was 10.7±1.4 g/dL, which was significantly lower than that of the normal group (P<0.05). The mean serum ALP level was 1,018.8±843.8 IU/L, which was significantly higher than that of the normal group (P<0.05). No child experienced hypocalcemia. The mean serum phosphate was 4.9±1.9 mg/dL, and hypophosphatemia (≤3.8 mg/dL) was observed in 2 children.
The mean serum iPTH level was 122.8±133 pg/mL, which was significantly higher than that of the normal group (P<0.05). The mean serum ferritin level was 20.5±24.3 ng/mL, which was not significantly different from the normal group. However, it tended to be lower than the normal group. The mean serum iron level was 26.2±20.2 ug/dL, and the mean transferrin saturation level was 5.4±2.2% which was lower than that of the normal group (P<0.05). IDA was more common in the deficient group than in the normal group (25.5% vs. 12.0%, P<0.05). In multiple regression analysis, young age and low Hb level were independent factors for vitamin D insufficiency (Figs. 1, 2).
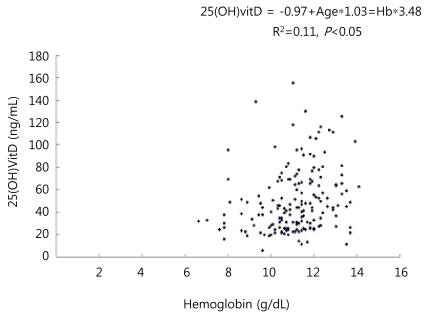
Linear correlation between vitamin D and hemoglobin level. 25-(OH)VITD, 25-hydroxyvitamin D; Hb, hemoglobin.
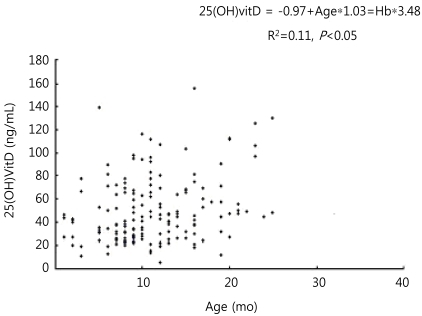
Linear correlation between vitamin D level and age. 25-(OH)VITD, 25-hydroxyvitamin D; Hb, hemoglobin.
Wrist radiographs were taken in 25 of 51 children in the insufficiency group, which demonstrated osteopenia 1 child, findings suggestive of rickets in 9 and normal findings in 15 children (Table 2). Of the 51 children, 11 had a 25-hydroxy vitamin D3 level of ≤20 ng/mL. In these 11 children, the mean body weight was 8.5 kg (SDS, -0.44±0.5 kg), and the mean height was 75.7 cm (SDS, -0.23±0.7 cm), which were remarkably lower than that of the normal and insufficiency groups. The mean serum Hb level was 10.4±1.5 g/dL, and the mean serum Hct level was 31.6±4.0%, which were significantly lower than that of the normal and insufficiency groups. Of these 11 children, 10 were breast-fed, 4 (36%) experienced concurrent IDA, with its frequency being significantly higher. In addition, there was a tendency for more severe findings suggestive of rickets in these 11 children.
3. Treatment outcome
1) Normal group
Of the 120 children, 10 (8%) were given calcitriol for a mean duration of 2.2 months. Their 25-hydroxyvitamin D3 levels were all at the lower normal limit and 5 of them showed radiological findings suggestive of rickets. Of these 10 children, 6 underwent follow-up 25-hydroxyvitamin D3 tests, in whom the D3 level increased form a mean pretreatment D3 level of 39.6±14.6 ng/mL to a mean post-treatment D3 level of 41.8±17.2 ng/mL. Three of these 10 children were followed up with radiographs: 2 showed improvement, 1 returned to normal.
2) Vitamin D insufficiency group
Of the 51 children, 18 (35.2%) were given calcitriol for a mean duration of 2.5 months. Other insufficient children were missed follow-up. Of these 18 children, 13 underwent 25-hydroxy vitamin D3 tests, and the D3 level increased from a mean pretreatment level of 20.7±7.0 ng/mL to a mean post-treatment level of 43.7±23.8 ng/mL. Of these 18 children, 7 were followed up with radiographs; 2 showed improvement, 2 returned to normal. Of the 11 children with a 25-hydroxy vitamin D3 level of ≤20 ng/mL, 4 (36%) were given calcitriol for a mean duration of 4.1 months. D3 level was markedly increased from a pretreatment D3 level of 13.3±5.2 ng/mL, to a mean post-treatment D3 level of 34.5±23.8 ng/mL. Of these 4 children, 2 were followed up with radiographs, both of whom returned to normal.
Discussion
In this study, the prevalence of vitamin D insufficiency is 29.8% of children younger than 2 years old. A recent large-scale study on the US population reported that 61% of 9,757 children experienced vitamin D deficiency, which is higher than that of our study. The prevalence of vitamin D deficiency was reported to be 3.1 to 57.8% in Chinese adolescents, 0.1% in Japanese children aged 1 to 4 years and 24% in Mongolian children younger than 1 year old18,21,22). These data are comparable to the outcomes of our study.
In this study, as age and serum Hb level decreased, serum vitamin D level also decreased (Figs. 1, 2). Grindulis et al.12) have demonstrated the relationship between vitamin D deficiency and IDA. Katsumata et al.23) have reported that IDA affects the synthesis and metabolism of iron-dependent enzymes for vitamin D activation, leading to osteopenia. Based on these results, serum vitamin D concentration should be measured in children with IDA, and vice versa. In this study, a considerable number of children with vitamin D insufficiency had concurrent IDA. It is suggestive that IDA associated with vitamin D insufficiency may affect bone growth. This suggestion is enhanced when considering that the height of vitamin D deficient children was found to be low, although their body weight was not significantly different from that of normal children. The reasons for lower vitamin D levels in younger children may be explained as follows. First, young children were beast-fed without vitamin supplement. Second, they undertook less outdoor activities than older children. Third, they experienced reduced exposure to sunlight, because they wore thicker clothes compared to older children while they were outdoors. To validate our results, we suggest further studies to investigate the proportion of breast-feeding in a cohort of mixed fed children, the duration of complete breast-feeding, the duration of sunlight exposure and the use of sun block.
The diagnosis rate of vitamin D insufficiency was highest in the spring in this study. The reason may be that duration of sunlight exposure is short during the winter. In addition, the lowest diagnosis rate of vitamin D insufficiency in the autumn may be explained by the fact that children were sufficiently exposed to sunlight during the summer. Vitamin D production requires sunlight, it is "a vitamin of the sun". However, parents are reluctant to expose their children to sunlight due to fear of skin cancer, or respiratory viral infection. The guidelines of the American Society of Pediatrics recommend that children aged less than 6 months should avoid direct sunlight exposure, and wear long clothing or use sun block while they are outdoors24). Reluctance to be exposed to sunlight due to concern about skin cancer or upper respiratory infection may be an important cause of the increasing prevalence of vitamin D deficiency all over the world.
Grindulis et al.12) have reported that in the winter, serum vitamin D concentration is significantly different between children with vitamin D deficiency who were given vitamin D supplement and those who were not. Whereas in the summer, it is not significantly different. Because Korea is situated in 34° to 43° North Latitude (Seoul, 37° 4') and sunshine duration is considerably shorter in regions situated above 37° North Latitude25). It is therefore necessary to perform vitamin D tests and supplement with vitamin D where appropriate, especially during the winter. Further studies are needed to determine whether vitamin D dosage should be adjusted according to the season.
We reported 8 breast-fed children with subclinical rickets10). It has been demonstrated that the risk of vitamin D deficiency rickets is higher in breast-fed infants11,22). In our study, the proportion of breast-feeding was higher in the vitamin D insufficiency group. Under the assumption that there is little sun exposure, children who ingest 750 mL of breast milk daily will in reality ingest 11 to 38 IU of vitamin D, which is much lower than the minimum requirement of 200 IU/day26-28). The American Society of Pediatrics guidelines recommend that infants aged less than 2 months should be given a daily vitamin D supplement of 400 IU, and their minimal annual vitamin D concentration should be maintained at ≥30 ng/dL29,30). In Korea, despite the fact that breast-feeding is increasing due to social emphasis on the advantage of breast milk, vitamin D tests and supplementation are still inadequately performed due to lack of understanding, and disagreement on criteria and timing for vitamin D tests and lack of pure vitamin D medication. Therefore, guidelines for these issues should be established to prevent children from becoming vitamin D deficient.
In this study, the annual frequency of hospitalization was higher in the vitamin D insufficiency group than in the normal group, albeit without statistical significance. Considering that immunological function of vitamin D, it is thought that children with vitamin D insufficiency are at high risk of serious diseases requiring hospitalization. Of 25 children in the normal vitamin D group who underwent radiological examination, 10 showed osteopenia and findings suggestive of rickets. These outcomes are suggestive that some children with normalized vitamin D concentration suffer from subclinical rickets characterized by persistent radiological changes of the bone. Additional studies on bone density and growth are needed to validate our findings. Curiously the mean level of ALP was high in the normal group. It is suggestive that there were also some children who have normalized vitamin D concentration suffer from subclinical rickets characterized by high level of ALP because of their compensative bone forming action. And another cause is some disease status may affect their ALP level in inpatients who did not meet exclusion criteria.
Limitation to this study include the following, although the relationship of the concentration with that of hemoglobin and transferrin saturation was positive, other factors reflect iron concentration were unrelated to the status of vitamin D. In addition, the number of children who have work up for IDA was too small (43 of 171).
In conclusion, this study demonstrated that approximately 30% of children aged ≤2 years experienced vitamin D insufficiency associated with subclinical rickets, and that vitamin D concentration decreased in the winter due to scanty sunlight. For these reasons, vitamin D insufficiency children should be given vitamin D supplement. In addition, vitamin D insufficiency can be associated with IDA and growth retardation, and breast-fed children are at high risk of vitamin D insufficiency. Thus, guidelines for vitamin D supplement in such children must be established. Since children with vitamin D insufficiency are also at high risk of serious diseases requiring hospitalization, and some continue to experience bony changes associated with morphological abnormalities, regular follow-up is important.