Novel influenza A (H1N1) 2009 infection in the pediatric patients with hematologic and oncologic diseases in the Yeungnam region
Article information
Abstract
Purpose
Natural history and consequences of the novel 2009 influenza A H1N1 (2009 H1N1) infection in immunocompromised pediatric patients are not yet fully understood. In this study, we investigated the clinical features and outcomes of the 2009 H1N1 infection in pediatric patients with hematological and oncological diseases.
Methods
We retrospectively reviewed the medical records of 528 patients who had hematological and oncological diseases and who were treated at 7 referral centers located in the Yeungnam region. Among the 528 patients, 27 with definite diagnosis of 2009 H1N1 infection were the subjects of this study. All patients were divided into the following 3 groups: patients who were receiving chemotherapy (group 1), patients who were immunosuppressed due to a non-malignant hematological disease (group 2), and patients who were off chemotherapy and had undergone their last chemotherapy course within 2 years from the influenza A pandemic (group 3).
Results
All 28 episodes of 2009 H1N1 infection were treated with the antiviral agent oseltamivir (Tamiflu®), and 20 episodes were treated after hospitalization. Group 1 patients had higher frequencies of lower respiratory tract infection and longer durations of fever and hospitalization as compared to those in group 2. Ultimately, all episodes resolved completely with no complications.
Conclusion
These results suggest that early antiviral therapy did not influence the morbidity or mortality of pediatric patients with hematological and oncological diseases in the Yeungnam region of Korea after the 2009 H1N1 infection. However, no definite conclusions can be drawn because of the small sample size.
Introduction
A novel swine-originating influenza A H1N1 virus spread rapidly around the world in 2009, and on June 11, 2009, the World Health Organization raised the pandemic alert level to phase 6, indicating that a global pandemic had begun1).
Initial reports suggested that illness associated with the pandemic 2009 influenza H1N1 (2009 H1N1) infection might be mild compared with the 1918 influenza pandemic2, 3). However, recently published series that included 122 children with confirmed 2009 H1N1 infection, reported a high prevalence of underlying medical conditions and described the need for intensive care and mechanical ventilation in 20% and 10% of patients, respectively4). In Korea, Shin et al.5) reported that high-risk pediatric patients had an elevated mortality rate following infection with novel influenza A (H1N1) virus.
In May, 2009, the first case of the 2009 H1N1 infection was identified in Korea. By February, 2010, 243 deaths had been reported6). Risk factors for severe complications of the 2009 H1N1 infection include chronic lung disease, heart disease, pregnancy, immunosuppression, and neurodevelopmental disorders3, 5, 7). Patients with hematological and oncological diseases are more susceptible to acquiring infections than the general population and potentially develop more complications. Because of disruptions in both innate and acquired immunity, even organisms with low virulence are able to cause significant morbidity and mortality8). There are several reports that the 2009 H1N1 infection in adults with hematological malignancies may have lead to severe complications9-11), although 49 adult patients with cancer and/or hematopoietic stem cell transplant (HSCT) who were diagnosed with the 2009 H1N1 infection had mild symptoms12). However, the reports on the natural history and the consequences of the 2009 H1N1 infection among the pediatric immunocompromised patients are very rare. Launes13) reported that the 2009 H1N1 infection in pediatric patients with acute lymphoblastic leukemia under maintenance treatment had a mild disease while children receiving intensive treatment had more severe diseases. Caselli et al.14) reported that the 2009 H1N1 infection in children with cancer was not the cause of death in any case.
This study was conducted to investigate the clinical features and the outcomes of the 2009 H1N1 infection in pediatric patients with the hematologic and oncologic diseases in the Yeungnam region of Korea.
Materials and methods
1. Patients
We retrospectively reviewed the medical records of the 528 patients under the age of 20 years who had hematological and oncological diseases and were treated at seven referral centers located in the Yeungnam region, between September, 2009 and February, 2010 (pandemic influenza A 2009). All patients were divided into three groups. Group 1 consisted of the patients who were receiving chemotherapy for malignancy during the study period (n=245). Group 2 consisted of patients who were immunosuppressed due to a non-malignant hematological disease, such as aplastic anemia, and those who had undergone a splenectomy for the treatment of hemolytic anemia (n=90). Group 3 consisted of patients who were off chemotherapy and had received their last chemotherapy within 2 years from the influenza A pandemic (n=193).
Of these 528 patients, 27 patients identified as having the 2009 H1N1 infections were the subjects of this study. None of them except one were vaccinated prior to 2009 H1N1 outbreak.
2. Methods
Laboratory diagnosis of the 2009 H1N1 infection was confirmed by the real-time reverse transcriptase-polymerase-chain-reaction assays (Exicyclor®96, Bioneer Inc, Alameda, CA, USA; Applied Biosystems 7500 Real-Time PCR System, Applied Biosystem, Foster City, CA, USA) of a nasopharyngeal swab. Upper respiratory tract infection was defined as the presence of pharyngeal congestion and/or rhinorrhea in the absence of lower respiratory tract symptoms, with normal chest X-ray findings. Lower respiratory tract infection was defined as the presence of the clinical findings such as coughing, hypoxia, dyspnea, wheezing, and rales with new pulmonary infiltrations on chest X-ray.
Laboratory data were collected and analyzed. Neutropenia was defined as an absolute neutrophil count (ANC) of less than 1,000/µL in infants between 2 weeks and 1 year of age and less than 1,500/µL beyond 1 year of age. Severe neutropenia was defined as an ANC of less than 500/µL. Lymphopenia was defined as an absolute lymphocyte count (ALC) of less than 1,800/µL in children under 2 years of age and less than 1,000/µL in children beyond 2 years of age.
3. Statistical analyses
Results are expressed as mean values, median values, and ranges. Statistical analyses were performed using Fisher's exact test, Kruskall-Wallis test, and the Mann-Whitney test. Significance was set at P<0.05. The SPSS (version 18.0, SPSS Inc, Chicago, IL, USA) was used for statistical analyses.
Results
1. Patients
Twenty-seven patients were diagnosed with the 2009 H1N1 infection and one patient had two separate episodes.
The median age of the 27 patients was 8±4.6 years and 15 (55%) were males. Ten patients (37%) were aged between 5 and 10 years old. Of the 28 episodes, 17 (60%) belonged to group 1, three (11%) belonged to group 2, and eight (29%) belonged to group 3.
Of the group 1 patients, 11 (41%) had leukemia, four (15%) had lymphoma, and one had Ewing sarcoma. A 6-month-old male infant was diagnosed with acute myeloblastic leukemia in November, 2009 and at the same time he was found to have the 2009 H1N1 infection. He received induction chemotherapy with the antiviral agent Tamiflu. Three patients in group 2 had non-malignant hematological diseases. Of the group 3 patients, six had leukemia and one patient each had lymphoma and neuroblastoma (Table 1).
Baseline Characteristics of Patients with Hematologic and Oncologic Diseases in the Yeungnam Region with Definite Diagnosis of 2009 H1N1 Infection
No patient had a preexisting chronic lung disease, congenital heart disease, or neurodevelopmental disorder.
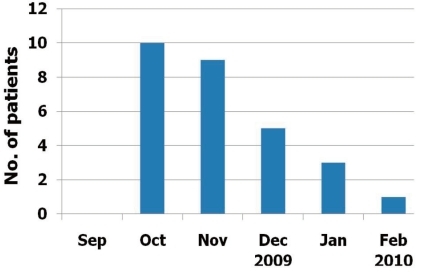
Ten episodes (35.7%) were confirmed in October, 2009 and nine (32.2%) in November, 2009. Similar distributions in the number of confirmed patients and in the number of patients with influenza-like illness of the Korean population reported by committees of the Korea Centers for Disease Control and Prevention were observed during the study period (Fig. 1).
2. Clinical presentations
The common symptoms presented at the time of diagnosis of the 2009 H1N1 were coughing and fever among all groups. Rhinorrhea/nasal stuffiness and sputum are the next common. The gastrointestinal symptoms were not seen other than vomiting (n=3, 11%). Neurological symptoms of headache were noted in 8 patients (28%) totally, and 4 cases in group 1. No statistical significant differences between groups were detected in the clinical features except duration of fever (Table 2). The mean duration of fever was 3.7±3.1 days in group 1, 2.7±0.6 days in group 2, 1.1±1.1 days in group 3, and totally 2.8±2.7 days. The mean duration of fever was significantly longer in group 1 (P=0.022).
3. Laboratory findings
Of the 28 episodes, complete blood cell counts were performed in 21 episodes. Laboratory studies were performed in all cases in group 1 and 2 (17 and 3 patients each), but only in one of the eight patients in group 3. Therefore, statistical comparison was not made. The median white blood cell count was 2.82×103/µL (range, 0.1-11.2×103/µL). Of the 21 episodes, leucopenia was revealed in 15 (71%), neutropenia in 11 (52%), severe neutropenia in 6 (28.5%). Lymphopenia was seen in 18 (85%) and the median ALC was 328/µL (range, 19-3,254/µL). Thrombocytopenia was noted in 12 (57%). Twelve (57%) had an elevated alanine aminotransferase (ALT) level (>45 IU/L) and aspartate aminotransferase (AST) level (>55 IU/L for children <10 years of age and >45 IU/L for children ≥10 years of age). C-reactive protein (CRP) was measured in 20 episodes, and the mean CRP level was 3.29±3.71 mg/dL and 19 (67.8%) had elevated levels (Table 3).
Initial Laboratory Findings of the Patients with Hematologic and Oncologic Diseases in the Yeungnam Region Confirmed with the 2009 H1N1 Infection
Chest X-rays were taken in 25 episodes; 11 (44%) had radiographic abnormalities with evidence of lower respiratory tract disease.
4. Outcomes
For 20 (71%) patients of total 28 episodes were treated after hospitalization. The median duration of the hospitalization was 8 (range, 3-87) days. In three cases, the durations of hospitalization were longer than 15 days. They had been newly diagnosed as Ewing sarcoma, lymphoma, and acute myeloblastic leukemia, and their duration of admission was prolonged for chemotherapy.
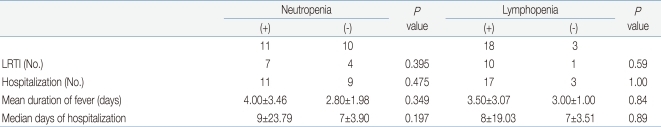
Significant differences between groups were detected in the frequencies of lower respiratory tract infection (P=0.03), admission (P<0.001), and durations of fever (P=0.022) and hospitalization (P=0.001). Group 1 had higher frequencies of lower respiratory tract infection and longer durations of fever and hospitalization (Table 4). Between the patients with lymphopenia and neutropenia, no difference was detected in the frequency of lower respiratory tract infection or duration of fever or hospitalization (Table 5).
All episodes were treated with oseltamivir (Tamiflu®, Roche, Basel, Switzerland) at a dose calculated based on patient body weight12). All treated patients tolerated Tamiflu well. Tamiflu was administered for a median of 1 day (range, 1-9 days) after onset of initial symptoms. Within a median of 1 day initial symptoms had subsided. As simultaneous bacterial infection could not be ruled out, 20 cases of hospitalized patients received systemic antibiotics in addition to Tamiflu treatment. Six cases received systemic antifungal agents, too. Of the six patients with severe neutropenia, one patient who received maintenance chemotherapy for acute lymphoblastic leukemia was treated with granulocyte colony-stimulating factor.
After recovery from 2009 H1N1 infection, one patient died of disseminated intravascular coagulation and septic shock 2 weeks later. Influenza infection was not directly responsible for any death.
Discussion
Seasonal influenza A infection has been associated with high morbidity and mortality in immunocompromised patients. In previous retrospective reports, seasonal influenza among patients with impaired immunity was associated with higher incidences of pneumonia and fatality. Chemaly et al.15) reported the overall mortality rate for community acquired respiratory viral pneumonia was 15% among HSCT recipients and patients with hematological malignancies. Mendoza Sanchez et al. noted that common respiratory virus infections in children with cancer or HIV infection have significant morbidity16). Pediatric oncology patients experienced significant influenza-associated morbidities17).
The clinical outcomes of the 2009 H1N1 infection in the pediatric patients with hematological and oncological diseases are not fully understood and are still being defined. Several studies have reported fatal cases in adults with cancers and in HSCT recipients9-11). Recently, Caselli et al.14) reported that the 2009 H1N1 infection in children with cancer was not the cause of death in any case; however, it resulted in reduced intensity of anti-cancer therapy.
In this retrospective study, we sought to investigate the clinical features and the outcomes of the 2009 H1N1 infection in the pediatric patients with hematological and oncological diseases who were potentially vulnerable to infections because of underlying immunosuppression. No patient required mechanical ventilation and no deaths were observed to be directly related to the 2009 H1N1 infections in this study. Most patients had mild disease and they recovered completely without complications related to the 2009 H1N1 infection.
When all episodes were grouped by disease status, statistically significant differences were observed between the groups in duration of fever and the hospitalization and development of lower respiratory tract infection. Most group 1 patients, who were on chemotherapy for their malignancy, had low lymphocyte and neutrophil counts at diagnosis of the 2009 H1N1 infection. Indeed, 10 patients had lymphopenia and nine had neutropenia. Previous studies in immunocompromised hosts with seasonal influenza found that lymphopenia was associated with prolonged shedding, a higher chance of the virus developing drug resistance during treatment, and pneumonia2, 11, 15, 18). We did not obtain information on viral shedding; however, our study shows that all patients with the 2009 H1N1 infections recovered clinically, regardless of lymphopenia or neutropenia.
These patients have been closely monitored and carefully treated. Because of the nature of the patients with hematologic and oncologic diseases, they were not readily vaccinated but tend to visit hospital earlier. Therefore, active treatment was possible at early stage of the disease.
This study had some limitations. The small sample size of this study does not allow for definite conclusions about the 2009 H1N1 infections in hematologic and oncologic pediatric patients. It is unclear whether patients treated in the Yeungnam region were representative of the greater population of hematological and oncological pediatric patients.
In summary, most pediatric patients with hematological and oncological diseases in the Yeungnam region, Korea, had favorable, and not fatal, courses from the 2009 H1N1 infection. As with the general population, coughing and fever were the most common symptoms. We conclude that pediatric patients with hematological and oncological diseases did not have increased morbidity or mortality following infection with H1N1 infection if they were treated with the antiviral agent Tamiflu during the early stages.