Change in the treatment strategy for pediatric Crohn's disease
Article information
Abstract
Crohn's disease is characterized by chronic inflammation involving any portion of the gastrointestinal tract. Treating Crohn's disease is a major challenge for clinicians, as no curative therapy currently exists. Pediatric Crohn's disease is characterized by frequent relapses, a wide extent of disease, a high prevalence of extraintestinal manifestations, and a severe clinical course. The classic therapeutic approach is known as the 'step-up' strategy, and follows a progressive course of treatment intensification as disease severity increases. Although this approach is usually effective for symptom control, many patients become either resistant to or dependent on corticosteroids. The efficacy of infliximab suggests that, rather than a progressive course of treatment, early intense induction may reduce complications associated with conventional treatment and improve quality of life. Intensive early therapy with infliximab is known as the 'top-down' strategy. Such therapy offers the potential for altering the natural history of Crohn's disease, and is changing treatment paradigms. However, the relatively new concept of an early aggressive or 'top-down' treatment approach is not yet widely accepted, especially in pediatric patients. The results of our current study demonstrate that early and intensive treatment of pediatric Crohn's disease patients with infliximab, at initial diagnosis, was more effective for maintaining remission and reducing flares.
Introduction
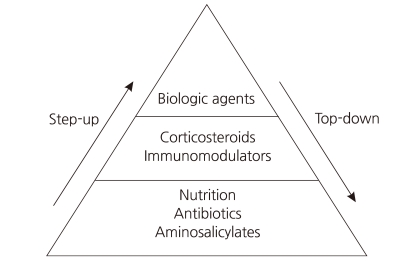
Crohn's disease (CD) is characterized by chronic inflammation involving any portion of the gastrointestinal tract. Although the etiology of CD remains incompletely understood, environmental factors, infectious microbes, ethnic origin, genetic susceptibility, and immune system dysfunction have been implicated in the associated chronic mucosal inflammation1). Treating CD is a major challenge for clinicians, as no curative therapy currently exists. The classic therapeutic approach is known as the "step-up" strategy, and follows a progressive course of treatment intensification as disease severity increases (Fig. 1).
Conventional therapy for active disease includes treatment with corticosteroids. Initially, corticosteroids are very effective and fast acting; however, long-term exposure to corticosteroids leads to drug dependency and/or resistance2). Several randomized, placebo-controlled studies have demonstrated that the biological drug infliximab (Remicade: Centocor Ortho Biotech, Malvern, PA, USA), a monoclonal immunoglobulin G1 chimeric antibody directed against tumor necrosis factor-α, is highly effective in improving the clinical course in patients with CD3-9). Since receiving FDA approval for pediatric use in May 2006, infliximab has been widely used in pediatric patients with CD10). The efficacy of infliximab suggests that, rather than a progressive course of treatment, early intense induction may reduce complications associated with conventional treatment and improve quality of life. Intensive early therapy with infliximab is known as the "top-down" strategy (Fig. 1).
Ethnic differences in the clinical characteristics of CD have been noted. CD is relatively common in developed countries, whereas it is not as common in Asian countries11). In addition, recent studies from Far East Asian countries reveal that the prevalence of NOD2 mutations, associated with increased susceptibility to CD in Western populations, is lower than that found in other populations12-14). Therefore, the clinical response to treatment for CD in Asian countries might differ from patients in Western countries.
Step-up strategy
Pediatric CD is characterized by frequent relapses, wide disease extent, high prevalence of extraintestinal manifestations, and severe clinical course15, 16). Current practice guidelines recommend that most patients with active disease should initially be treated with corticosteroids17, 18). Although this approach is usually effective for symptom control, many patients become either resistant to or dependent on these drugs19). Long-term exposure to corticosteroids is also associated with complications such as Cushing's syndrome, and therefore, with increased risk of mortality17, 18, 20-22). For this reason, most clinicians initiate treatment with corticosteroid-sparing drugs such as azathioprine, mercaptopurine, or methotrexate once corticosteroid resistance or dependence develops.
Infliximab is an anti-tumor necrosis factor (TNF-α) monoclonal antibody that was initially used in corticosteroid-dependent and corticosteroid-refractory CD23). TNF-α is a pro-inflammatory cytokine that plays a key role in the pathophysiology of a number of inflammatory disorders, including CD. The use of infliximab has been well documented as an effective treatment for moderate to severe CD in children in several studies24, 25). Infliximab, as a scheduled treatment, is effective for the induction and maintenance of remission in patients with CD26, 27) (Fig. 1).
Top-down strategy
Early use of infliximab with immunosuppressants, known as top-down therapy, in patients with newly diagnosed CD has resulted in better outcomes in adult patients28). An 8-week maintenance treatment schedule with infliximab has been shown to be a cost-effective approach in adult patients with active luminal or fistulizing CD29). Such therapy offers the potential for altering the natural history of CD, and is changing treatment paradigms. One possible explanation for the prolonged remission with infliximab treatment is that this medication might help heal mucosal lesions in patients with severe disease as well as those with mild disease30). However, the relatively new concept of an early aggressive or top-down treatment approach is not yet widely accepted, especially in pediatric patients, even although some studies have reported that infliximab is more effective in children than in adults31, 32) (Fig. 1).
The safety of infliximab
The safety profile of infliximab is an important issue, as its use and indications are rapidly increasing. Infliximab treatment appears to be well tolerated7), however, anaphylactic reactions and symptoms caused by immunosuppression, such as upper respiratory tract infections, herpes zoster activation, severe acute bacterial infections, and an increased risk of tuberculosis, have all been described following treatment with infliximab8, 9, 33). Pretreatment before infliximab infusion may be helpful in ameliorating these side effects. Little is known about the potential for long-term toxicity in infliximab therapy. After repeated dosing is performed, some patients have human antibodies to chimeric TNF-α antibodies34). Repeated administration of chimeric antibodies may be associated with the formation of autoimmune antibodies and a self-limited lupus-like syndrome35).The development of lymphoma has been reported as a possible, although rare, long-term complication of anti-TNF therapy in patients with rheumatoid arthritis and CD36-38). Several reports of a possible association between concomitant infliximab and immunomodulator therapy and hepatosplenic T-cell lymphoma (HSTCL) have emerged37, 38). However, the mechanism of this possible association remains unclear. Until further evidence emerges, long-term surveillance of patients who have used infliximab in combination with immunomodulators is warranted.
The experience in Samsung Medical Center
We have used infliximab as an induction therapy since 2005. Our patients had a better response to infliximab treatment than compared to conventional therapy. At 8 weeks, remission was achieved in 3 of 11 patients (27.3%) in the step-up group, and in 16 of 18 patients (88.9%) in the top-down group. At 1 year of follow-up, remission was maintained in 5 of 11 patients (45.5%) and in 15 of 18 patients (83.3%) in the step-up and top-down groups, respectively39). At the 1 year follow-up, the relapse rate (23.1%, 3 of 13 patients) in the top-down group was lower than that (61.5%, 8 of 13 patients) in the step-up group (P=0.047). At 2 years follow-up, the relapse rate (38.5%, 5 of 13 patients) in the top-down group was lower than that (76.9%, 10 of 13 patients) in the step-up group (P=0.047)40). The mechanism(s) by which the top-down strategy yields superior results when compared to step-up treatment with regard to remission rates remains unclear. However, it is suggested that the patients in the step-up group had a much longer duration of disease and that they were exposed to more extensive tissue damage. Limbergen et al.15) reported that infliximab treatment early in the disease course may avoid the irreversible tissue damage often found in later stages of CD. The potential of infliximab to not only induce but also maintain remission is now well studied in adult and pediatric CD cohorts5, 6, 24).
Conclusion
The incidence and prevalence of CD in Korea is lower than in developed countries; however, it is rapidly increasing. There is no known cure for CD. Therefore, the goal of treatment is to mitigate inflammation and the associated clinical symptoms. The current treatment guidelines are designed to maintain remission after induction therapy.
According to our experience, early and intensive treatment of pediatric CD patients with infliximab, at initial diagnosis, was more effective for maintaining remission and reducing flares.