Increased B cell-activating factor (BAFF) level in the sputum of children with asthma
Article information
Abstract
Purpose
B cell-activating factor (BAFF) is a tumor-necrosis factor (TNF) superfamily member best known for its role in the survival and maturation of B cells. BAFF activity is observed in naïve cells as well as in effector/memory T cells. We aimed to explore whether BAFF in sputum is expressed at elevated levels in asthmatic airways and associated with eosinophilic inflammation, pulmonary function, and bronchial hyperresponsiveness in children.
Methods
One hundred and fifty-four asthmatic children and 98 healthy children were enrolled in the study. Sputum supernatants were collected and sputum BAFF and eosinophil cationic protein (ECP) levels were measured. We performed pulmonary function tests and methacholine challenge tests, while measuring total eosinophil count, total serum IgE, and serum ECP in all subjects.
Results
Asthmatic children had significantly higher levels of BAFF in induced sputum [26.50 (10.50-100.27) pg/mL] compared to healthy children [18.32 (7.68-44.63) pg/mL; P=0.011]. Sputum BAFF positively correlated with sputum eosinophils (γ=0.406, P<0.001) and sputum ECP (γ=0.789, P<0.001). Significant negative correlations were found between sputum BAFF and FEV1 (γ=-0.291, P<0.001) or post-bronchodilator FEV1 (γ=-0.334, P<0.001), whereas nonsignificant correlations were found between sputum BAFF and bronchial hyperresponsiveness, serum eosinophil count, and serum ECP.
Conclusion
These findings suggest that BAFF may play a role in childhood asthma, and BAFF levels in sputum could be a supportive marker that represents airway inflammation, especially eosinophilic inflammation.
Introduction
Asthma is a chronic inflammatory disease in the airway that causes reversible or irreversible contraction of the airway and causes sudden dyspnea through a complex inflammatory reaction that includes allergic reactions. Asthma occurs from the interaction of genetic factors and environmental factors. One of the most important pathophysiologies in asthma, however, is an allergic inflammation that is caused by persistent exposure to allergens1). When an allergen is recognized by Th2 cell, cytokines such as IL-4 and IL-5 that promote the maturation and differentiation of the B cell are secreted due to the activation of the Th2 cell. These cytokines induce the B cell to be differentiated into a plasma cell and to produce the immunoglobulin E (IgE) antibody that has an important role in allergic inflammation2).
B cell-activating factor (BAFF), also known as BLys, TALL-1, zTNF4, or THANK, is part of the tumor necrosis factor family and is known as having an important role in differentiating the B cell and maintaining the shape of a mature B cell. In particular, BAFF is an important factor of the proliferation, differentiation, and survival of an undifferentiated B cell in the spleen3-5). A previous study reported that the B cell in the follicular zone and the marginal zone was not found in a BAFF-deficient mouse, which indicates that BAFF is an essential factor of the homeostatic expression, differentiation, and proliferation of the B cell6, 7). The high concentration of BAFF was clinically measured in patients with autoimmune diseases that show the over-differentiation of the B cell such as in rheumatic arthritis, autoimmune diabetes, Sjögren's syndrome, and multiple sclerosis7, 8). The activity of BAFF was also observed in the T cell9), and it was further found that BAFF affects the regulation of the interaction between an antigen-presenting cell and the T cell4). Based on the results of a previous study, several studies have been recently conducted to identify the detailed mechanism in which BAFF has a certain role in the allergic reaction that is a multiple immune response of the T cell and the B cell10-12). They reported that the BAFF concentration in the serum in patients with asthma was higher than in the normal group10), but that there was no difference between the patients with atopic dermatitis and the normal group11). Therefore, the exact role of BAFF in allergic diseases is not yet known.
In this study, the level of BAFF in the sputum of patients with asthma, a typical allergic disease, was measured to investigate its clinical significance and correlation. In addition, its associations with conventional asthma-related factors and pulmonary function variables were investigated.
Materials and methods
1. Subject
Two hundred and fifty-two children who visited Severance children's hospital were investigated. Of the 252 children, 154 subjects who were diagnosed with asthma were assigned to the patient group, and 98 subjects were assigned to the control group. According to the guidelines of the American Thoracic Society, asthma was defined as the cases of the pediatric patients who visited the hospital within the past 12 months due to cough without cold, wheezing, and dyspnea, in whom bronchial hyperresponsiveness (PC20<16 mg/mL) was observed in a methacholine bronchial challenge test or whose forced expiratory volume in one second (FEV1) after inhalation of the β2 agonist increased by 12% from the pre-inhalation state, in which case the reversibility of the condition was confirmed13, 14). Of the patients with asthma, 76 received treatment for asthma, including the inhalation of steroids in the past four weeks, whereas 78 did not receive any treatment. The control group was defined as those with no cases of wheezing, no medical history of other chronic diseases, and no medical history of infection within the past two weeks, and, among the subjects who visited the hospital for a health examination or a vaccination, those who did not show bronchial hyperresponsiveness in the methacholine challenge test. This study was approved by the Institutional Review Board of Severance Hospital and informed consent was obtained from the children's parents.
2. Method
1) Collection and treatment of sputum
The induction and collection of sputum was performed according to the protocol that Yoshikawa et al. reported15). After all the subjects were asked to wash their mouth with tap water, sputum was collected from them by inducing coughing at three-minute intervals after their inhalation of 3 mL of 3% saline solution for 10-20 minutes using an ultrasonic nebulizer (NE-U12; Omron Co., Tokyo, Japan). The pulmonary function was measured each time after the sputum induction. If FEV1 decreased to below 70%, the induction was halted. The sputum induction was resumed after FEV1 returned to its baseline value. The collected sputum was immediately treated within 2 hours or stored at 4℃ to minimize cell destruction. Subsequently, after the sputum was diluted five times with PBS that included 10 mmol/L of dithiothreitol (WAKO Pure Chemical Industries, Ltd., Osaka, Japan), it was stirred with a vortex mixer at room temperature for 20 minutes and then centrifuged at 2,000 rpm once the mucous component had melted. The suspension was stored at -70℃, and the precipitate was smeared on a slide using cytospin (Cytospin3: Shandon, Tokyo, Japan), followed by May-Grünwald-Giemsa staining. The percentages (%) of the neutrophils, lymphocytes, macrophages, and eosinophils were calculated after the slides that had 400 or more non-squamous cells were chosen. The numbers of the cells were counted using a hemocytometer (BLAUBRAND® counting chambers, Wertheim, Germany). The survival rates of the cells were measured after the cells were stained with a 0.4% trypan blue solution, and cells with survival rates lower than 70% were excluded from the results.
2) Pulmonary function test and methacholine challenge test
A pulmonary function test was performed using spirometry (Vmax encore, VIASYS Healthcare, Inc., Hoechberg, Germany) according to the standards of the American Thoracic Society14). In the three trials that were performed at each time point, the maximum FEV1 was selected. A methacholine challenge test was performed after it was confirmed that the FEV1 was more than 70% of the normal estimated value in the pulmonary function test16). In the methacholine challenge test, after methacholine was dissolved in a saline solution buffer and diluted to 0.075, 0.15, 0.31, 0.62, 1.25, 2.5, 5, 10, 25, and 50 mg/mL concentrations, the test was performed using a Rosenthal-French dosimeter (Ferraris, Hertford, England) via inhalation of methacholine with an aerosol through a Devilbiss 646 nebulizer for 0.6 seconds. Sixty to 90 seconds after the inhalation of increasing concentrations of methacholine at t5-minute intervals, FEV1 was measured. This process was repeated with increasing methacholine concentrations until the FEV1 decreased by more than 20% from the baseline value. The methacholine concentration at the point at which FEV1 decreased by 20% (PC20) in the dose-response curve was calculated. Bronchial hyperresponsiveness was defined as that in a case in which the PC20 was 16 mg/mL or less. The administration of an anti-inflammatory drug and a bronchodilator was halted 24 hours before the test.
3) Measurement of the number of eosinophils, total IgE, and the eosinophil cationic protein (ECP) in the serum, ECP, and BAFF in the sputum
The number of eosinophils in the blood was counted using the NE-8000 system (Sysmax, Kobe, Japan) after the collection of peripheral blood. The total IgE in the serum and the total blood ECP and sputum ECP were measured using the CAP radioallergosorbent technique (UniCAP; Pharmacia and Upjohn, Uppsala, Sweden). The sputum BAFF was measured using commercial ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA). The minimum concentration of 16 pg/mL was measured. The concentration of sputum BAFF was measured using a microplate reader (VERSA max) twice at OD450 nm, and the mean values were analyzed.
3. Statistics
The mean±SD was used when the variables (the number of eosinophils in the blood, the total IgE in the serum, the ECP, the sputum ECP, BAFF, and PC20) were normal, and the median (interquartile range) was used when the variables were abnormal. The student's t-test or the Mann-Whitney test was used to compare the asthma group with the control group. The correlations of the sputum BAFF's concentration with the pulmonary function variables and PC20 were calculated using Pearson's correlation test. The abnormal variables were converted into common logarithms and analyzed. They were considered statically significant when their P value<0.05. SPSS version 13.0 (SPSS, Inc., Chicago, IL) was used for all the statistical analyses.
Results
1. Characteristics of the pediatric subjects
The study subjects were a total of 252 children aged 8.76±2.43 years, of whom 159 (63.1%) were male and 93 (36.9%) were female. 154 of the subjects belonged to the asthma group, and 98, to the normal control group. The mean values of the numbers of eosinophils in the sputum and eosinophils in the blood, as well as of the total IgE and ECP, were more statically significant in the asthma group than in the control group (P<0.001, Table 1).
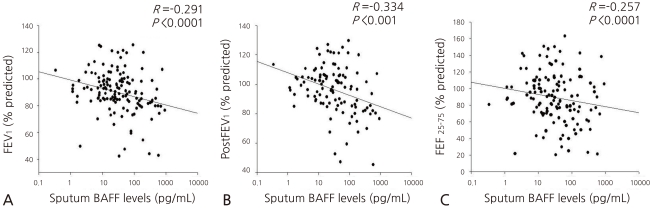
2. Comparison of the two groups' BAFF concentrations in the sputum
The concentration of the sputum BAFF was significantly higher in the pediatric patients with asthma than in the normal group [26.50 (10.50-100.27) pg/mL vs. 18.32 (7.68-44.63) pg/mL, P=0.011, Fig. 1A]. When the pediatric patients with asthma were divided into two groups, the pre-treatment group who were subjected to steroid inhalation (n=74) and the post-treatment group (n=76), it was found that the concentrations of the sputum BAFF in the pre-treatment group were higher than those in the post-treatment or normal group [33.60 (9.79-113.56) pg/mL vs. 23.86 (10.76-78.48) pg/mL vs. 18.32 (7.68-44.63) pg/mL, P=0.003 and P=0.036, respectively]. The difference in the concentrations of the sputum BAFF of the pre- treatment group and the post-treatment group (P=0.356) was not statistically significant, though (Fig. 1B).
(A) Sputum B cell-activating factor (BAFF) levels in asthma and control groups. Asthmatic children had significantly higher levels of BAFF in induced sputum [26.5 (10.5-100.3) pg/mL] compared to healthy children [18.3 (7.7-44.6) pg/mL; P=0.011]. (B) Sputum B cell-activating factor (BAFF) levels in asthma with/without treatment and control groups. BAFF levels of asthmatic group before treatment were higher than the levels after treatment in the control group [33.60 (9.79-113.56) pg/mL vs. 23.86 (10.76-78.48) pg/mL vs. 18.32 (7.68-44.63) pg/mL, P=0.003, P=0.036, respectively).
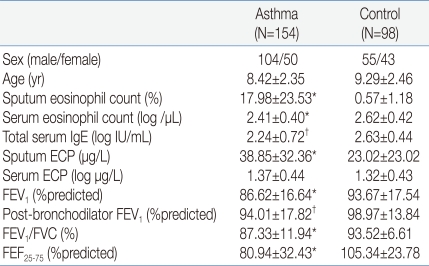
3. Correlation of the BAFF concentration in the sputum with ECP and the number of eosinophils in the sputum
The sputum BAFF showed significantly positive correlations with the sputum ECP (γ=0.406, P<0.001) and the number of eosinophils (γ=0.789, P<0.001), respectively (Fig. 2A, B). It showed no statistically significant correlation, however, with the total number of eosinophils, ECP, and IgE concentration in the blood.
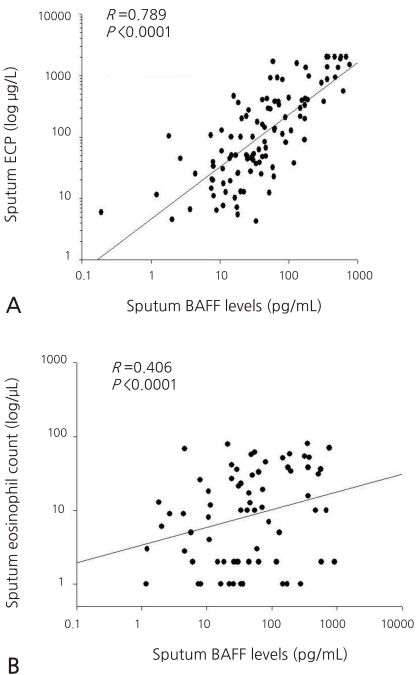
4. Correlation of the BAFF concentration in the sputum with the values from the pulmonary function test
The concentration of the sputum BAFF showed significantly negative correlations with FEV1 (γ=-0.291, P<0.001), FEV1 (γ=-0.334, P<0.001), and FEF25-75 (γ=-0.257, P<0.001) after the inhalation of a bronchodilator in the pulmonary function test (Fig. 3A, B, and C). No statistically significant correlation with PC20 and FEV1/FVC was found, though.
Discussion
In this study, it was shown that sputum BAFF is involved in the pathophysiology of asthma after it was seen that the concentration of the sputum BAFF was higher in the asthma group than in the normal group and that the concentration of the sputum BAFF had no positive correlation with allergic inflammation markers such as the number of eosinophils and ECP.
BAFF has been known as a substance involved in the maturation and differentiation of the B cell. It was recently reported as also being involved in the immune response of the T cell. BAFF's role with respect to the T cell seems to apply not only to a naïve T cell but also to a differentiated T cell4). Therefore, besides BAFF's role with respect to the B cell immune disease or the autoimmune disease that were revealed previously, it is also expected to play a certain role in diseases related to the T cell. In addition, as BAFF's involvement in IgE class switching in the B cell regulation process has yet to be identified, it is presumed that BAFF has an impact on the mechanism of allergy outbreak. In recent studies, Kang et al10) found that the concentration of the serum BAFF in pediatric patients with asthma increased, and that such increase is associated with the severity of the asthma. Thus, they suggested that BAFF should be used as a marker of the severity of asthma. Moon et al12) also reported that the symptoms of asthma disappeared when the concentration of the serum BAFF decreased. There have been reports, however, that BAFF is irrelevant to allergic diseases11). One study reported that allergic airway inflammation was suppressed in an asthmatic mouse model with high BAFF expression, based on which the study proposed that BAFF should not be a causative substance or a signaling substance but an outcome substance of inflammatory reactions. That is, BAFF should be a substance that induces the inhibitory mechanism of allergic inflammation17). Although recent studies have reported that the serum BAFF is associated with asthma, no study has presented the exact mechanism involved. Furthermore, most study results analyzed serum BAFF without determining the association of asthma with sputum BAFF.
Asthma is a disease in which reversible airway obstruction and airway remodeling occur due to the long-term persistent allergic inflammation of the airway. Not only lymphocytes such as B cells and T cells, but also various types of inflammatory cells that exist in the airway such as airway epithelial cells, eosinophils, and mast cells, are involved in the airway inflammation18). Accordingly, induced sputum has been used well to measure the degree of inflammation both in asthma and other chronic airway inflammatory diseases. In addition, as the measurement of the number of eosinophils in the sputum helps in the measurement of the change and severity of diseases, the measurement of the number of eosinophils using induced sputum is being used widely as a non-invasive method of monitoring asthma conditions19). In this study, the BAFF concentration in induced sputum was measured to investigate its association with asthma compared with that of serum BAFF. The measurement showed significant results.
Eosinophil plays an important role in allergic airway inflammation, and ECP, a major product of eosinophil, is used as a marker of allergic inflammation. The result of this study, in which the sputum BAFF showed strong positive correlations with the eosinophils and ECP in the sputum, supports the hypothesis that sputum BAFF is associated with asthma and is involved in allergic inflammation. Interestingly, the sputum BAFF in the pediatric patients with asthma showed no positive correlation with the number of eosinophils and the ECP in the serum. Eosinophils in the airway directly damage the airway epithelial cells by secreting various mediators, including cytokines. Therefore, the eosinophils in the airway are thought to be more closely associated with asthma than the eosinophils in serum20). This finding likely explains why sputum BAFF has a higher correlation with the eosinophils in the sputum than in serum.
The correlation of BAFF with the pulmonary function in the asthma group showed a negative correlation with FEV1, but no significant correlation with PC20 was found. When the BAFF concentrations of the pre-treatment and post-treatment groups in the asthma group were compared, however, it was seen that the BAFF concentrations before the treatment were significantly higher than after the treatment. Based on this result, it seems that sputum BAFF is associated with the recent treatment or worsening of asthma in patients. In this study, the severity of the asthma in the asthma group was not classified for analysis. The degree of the symptoms and the association with BAFF will be more clearly revealed in the future through the classification of the severity of the condition of the asthma group.
There was no statistical significance between the IgE concentration and the BAFF concentration in the sputum. This result contradicts the well-known mechanism by which BAFF induces an increase in IgE. As in several previous studies that showed the correlation of serum BAFF with allergic diseases10, 11), however, the concentration of IgE and BAFF did not always show a positive correlation in this study. As a result, it is suggested that BAFF should not be considered a starting substance of inflammatory reaction but an outcome or an inhibitor of inflammatory reaction. A further study is required to explore this suggestion.
In conclusion, this study confirmed that sputum BAFF is associated with asthma and has positive correlations with markers of allergic airway inflammation such as the number of eosinophils and ECP. This study is clinically significant because it showed that BAFF is associated with causes of asthma. A further study on the mechanism of BAFF in allergic diseases is needed, though.