The effect of sucrose on infants during a painful procedure
Article information
Abstract
Purpose
The purpose of this study was to test the efficacy of treating the pain among newborn infants associated with a medical procedure with sucrose with regard to overall physiological and behavioral stability.
Methods
103 newborn infants were enrolled in this study. The control group (n=63) did not receive any treatment. The experimental group (n=40) received 2 mL of 24% sucrose solution two minutes before a routine heel stick. The pain was assessed by measurements of physiological changes [e.g. pulse rate, oxygen saturation, salivary cortisol (hydrocortisone)] and behavioral changes [e.g. crying time, and the neonatal infant pain scale (NIPS) for neonates].
Results
There were no differences among the groups with respect to physiological changes associated with the pain from the procedure. However, there were significant group differences in behavioral changes to the pain. In the control group, the median crying time was 13 seconds, while in the experimental group, the median crying time was 3.5 seconds (P=.000). In the control group the median NIPS score was 4, while in the experimental group the median NIPS score was 2 (P=.000).
Conclusions
These findings suggest that sucrose can be an effective method for the management of stress responses in infants with regard to behavior. However, this treatment had no significant physiological effects.
Introduction
Treating pain in the newborn is essential for many reasons: pain can be harmful due to decreased oxygenation, hemo-dynamic instability, and increased intracranial pressure1). The International Evidence-Based Group for Neonatal Pain recommends that the combination of a variety of pharmacological and behavioral interventions during painful procedures has synergistic effects2). Recent studies have shown that the combination of oral sucrose and a pacifier was the most clinically safe and effective method for the management of painful procedures in neonates3-5).
Safety and efficacy are the major concerns in the selection of an appropriate pain-relieving treatment in infants2, 6). According to pain management recommendations7), newborn infants over 1,000 g in weight may be given a 24% sucrose solution orally (0.5-2 mL) via a nipple or syringe. However, administering sucrose using a pacifier is more natural than the use of a syringe and is clinically safer, lessening the chances of iatrogenic stresses and aspiration.
Salivary cortisol has been measured in infants subjected to major stressful situations, such as painful procedures, making it a useful alternative to blood sampling; the salivary cortisol level is a reasonable reflection of the hypothalamic-pituitary-adrenal axis function8, 9). Although circulating cortisol in the early weeks of life can be affected by an intrinsic transient productive failure because of immaturity of the hypothalamic-pituitary-adrenal (HPA) axis10), cortisol levels are stabilized by day 3 following birth11). We have also reported on the salivary cortisol responses in newborn infants to the stress at that critical time12).
However, other studies did not demonstrate the effects of oral sucrose on salivary cortisol changes during painful procedures in newborn infants13, 14). So, more explanative studies concerning the effects of sucrose on newborn infants by identifying salivary cortisol changes are needed.
Most studies concerning the effect of sucrose on infant pain have mainly evaluated partial biomarkers3, 4, 7, 14). Little is known about the effects of oral sucrose on the overall physiological and behavioral stability of newborns during painful procedures, although infant pain is a multidimensional phenomenon6).
In the current study, pre-and post-heelstick stress response patterns were compared between a routine procedure and a routine procedure with orally administered sucrose solution by observing physiological (pulse rate, oxygen saturation, and salivary cortisol) and behavioral (crying time and NIPS score) changes. To our knowledge, this is the first prospective study conducted in Korea on the effect of sucrose on multidimensional aspects of the pain response of newborn infants including salivary cortisol.
Materials and methods
1. Subjects
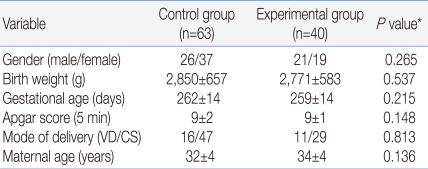
The study was conducted at Chonbuk National University Hospital, Jeonju, South Korea, between July 2008 and September 2009. The study was reviewed and approved by the Research Ethics Committee of Chonbuk National University Hospital. Subjects were assessed for eligibility and consent from the parents was obtained in 143 infants. Forty infants were excluded because the saliva volume was too small to analyze. Finally, 103 infants (control group n=63; experimental group n=40) were evaluated. All infants were healthy and had no congenital malformations. The groups did not differ significantly in any of the demographic variables (Table 1). The parents were awarded a $3.00 honorarium for their participation.
2. Data collection
Physiological and behavioral pain indicators were examined to maximize the validity of pain assessment in newborn infants. Data were collected during a heelstick performed between day two and seven day after delivery as part of routine clinical care. The infants in the control group (n=63) did not receive any treatment. In the experimental group, one author administered the sucrose solution (24%, 2 mL) 2 minutes prior to the heelstick procedure using a Similac premature nipple & ring (Abbott Nutrition, Columbus, Ohio, USA). It took 3-5 seconds for infants to consume all of the sucrose solution. After sucrose administration, infants self-expelled the pacifier, which was not reapplied. The pulse rate, oxygen saturation, and salivary cortisol were evaluated postnatally after stabilization of birth-related fluctuations (between 72 and 144 hrs after birth)8, 10, 11) during a routine heelstick procedure conducted for metabolic screening of the newborns. Data collections as well as saliva sampling were performed only between 7-8 AM during 72-144 hrs postnatally, before (pre-heelstick) and 25-30 min post-heelstick. The time of the collection was based on the results of experiments showing the peak cortisol responses between 20 and 30 min post-manipulation9). The neonates were not fed for 1 hrs preceding saliva sampling. Saliva was collected using commercial swabs designed for infant use (Sorbette®; Sarstedt, Rommelsdorf, Germany). Baseline levels were obtained in an undisturbed environment. None of the babies cried or showed signs of discomfort during the collection of saliva. Disposable pulse oximetry probes were applied to the infant's wrist and calibrated oxygen saturation was monitored using a Nellcor N-560 monitor (Nellcor Puritan Bennett LLC, Boulder, CO, USA) . The pulse rate and oxygen saturation data were manually recorded from the monitor every 15 sec for two minutes during the resting baseline phase and just after the heelstick phase.
The behavioral pain was assessed by measuring the crying time with a stopwatch and the pain assessment tool, NIPS, which is frequently utilized as an outcome measure for investigation of acute procedural pain in term and preterm neonates2, 15). The NIPS score is rated on the expression of the face (0, 1), cry (0-2), breathing patterns (0, 1), arms (0, 1), legs (0, 1), and state of arousal (0, 1). The total score ranges from 0 to 7. A score greater than three indicates clinically significant pain15). Data were carefully collected and analyzed without the preconception of group assignments.
Saliva samples were sealed and frozen (from -15 to -20℃) immediately after collection. And these samples were shipped with icebox frozen in dry ice to the Green Cross Reference Lab, (Seoul, Korea) for determination of salivary cortisol concentrations using an enzyme immunoassay with an ER HS Salivary Cortisol kit (Salimetrics, State College, PA, USA). Saliva samples were kept at - 70℃ for one or two months until analysis.
Statistical analyses were performed using the statistical package SPSS for Windows (version 15.0, SPSS Inc, Chicago, IL, USA). Comparison of demographic factors was analyzed using the mean, standard deviation, frequency, χ2 test and t-test. The differences in the pulse rate, oxygen saturation, and salivary cortisol were compared between pre- and post-heelstick by paired t-test. Mean values of these data were compared between groups using the repeated measures analysis of variance (ANOVA). Crying time and NIPS between two groups were compared with Mann-Whitney U test.
Results
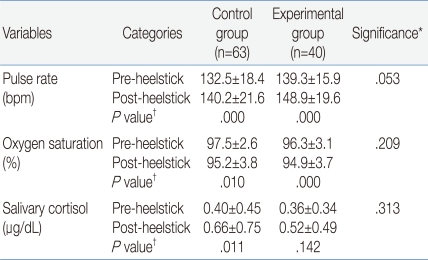
The demographic and clinical characteristics of the 103 infants are shown in Table 1. The heelstick procedure was stressful to infants based on the physiological responses (Table 2) and behavioral expressions (Fig. 1, 2). There were no significant differences between groups in the physiological responses. For the control group, there was about 8 beats per minute (bpm) increase in the pulse rate and in the experimental group there was about 10 bpm increases in the pulse rate. In the control group there was about two percent decrease in oxygen saturation and in the experimental group there was about one percent decrease in oxygen saturation.
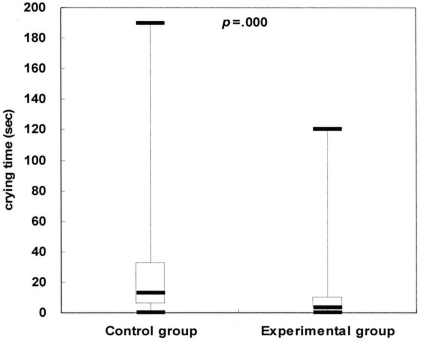
Comparison of crying time between the control and experimental groups. The line inside the boxes represents the median value, the lower border of the box represents the border of the 25th percentile, and the upper border of the box represents the upper border of the 75th percentile of the observations.

Comparison of the neonatal infant pain scale (NIPS) scores between the control and experimental groups. The line inside the boxes represents the median value, the lower border of the box represents the 25th percentile, and the upper border of the box represents the upper border of the 75th percentile of the observations.
The noticeable physiological difference in individualized groups was the salivary cortisol change from pre- to post-heelstick. In the control group there was about 0.26 g/dL increase in salivary cortisol (P=.011), while in the experimental group there was about 0.16 g/dL increase in salivary cortisol, which was not statistically significant (P=.142). The results indicated that sucrose administration attenuated the salivary cortisol increase in the experimental infants, although salivary cortisol changes were not statistically significant in the two groups (P=.492).
However, there were significant differences in the behavioral responses to pain. In the control group the median crying time was 13 sec, while in the experimental group the median crying time was 3.5 sec (P=.000). In the control group the median NIPS score was 4, while in experimental group the median NIPS score was 2 (P=.000).
Discussion
In this study, the heelstick collection of blood produced increases in the pulse rate and cortisol and a decrease in oxygenation. The pulse rate and oxygen saturation results are consistent with previous observations following heelstick procedures16). The present findings showed that the pulse rate and oxygen saturation of the experimental group was not different from the control group.
The level of salivary cortisol increased in the infants after the heelstick, although a wide range of variation was observed. However, variable cortisol levels have been shown in other studies as well9, 17). These findings support the view that the heelstick procedure is a significant stressful event for a newborn, altering salivary cortisol levels9).
This study represents a noteworthy attempt to identify salivary cortisol changes in newborn infants at 3-7 days using sucrose as a method of pain relief. Even though there were no significant differences in the salivary cortisol levels between the two groups, salivary cortisol changes in the experimental group were diminished in comparison to the control group. There would be two meaningful hypothetical thinking. First, divergent changes in salivary cortisol demonstrate the complexities in responses of newborn infants. Previous studies found that newborn infants adapt to extrauterine life as manifest by the maturation of the adrenal cortex and production of endogenous cortisol after birth10, 18). Second, the salivary cortisol is not a useful stress marker in the infants. The opinion might be supported by Gunnar et al19). They reviewed studies according to the relationships the cortisol and stress in childhood within last 15 years: some of them were significantly elevated in stressful situations while others were not significant. Then they suggested that the neural systems regulating the development of HPA reactions to stressors are being shaped by an interaction of genes and experience over early years.
The results of our study showed that the sucrose solution resulted in lower pain scores; the median NIPS score of the experimental group was lower than 3, and there was less crying time than in the control group. This finding suggests that the administration oral sucrose by pacifier was clinically safe and effective as a method for management decreasing procedure related pain in neonates, at least with regard to the behavioral aspects3-5, 7, 20). Previous studies support the theory that sucrose and pain relief are associated through the body's endogenous opioid system that provides natural analgesia. In a study on rats, oral administration of sucrose caused a significant elevation in pain thresholds5).
In summary, sucrose solution can be a handy and appropriate method to use for pain relief in infants with regard to behavior; while it was not found to have significant physiological effects including the salivary cortisol levels.
Acknowledgements
We thank families who participated in this project and the staffs at neonatal intensive care unit of the Chonbuk National University Hospital for their assistance during data collection. We are also most grateful to technicians at Green Cross Laboratory for analyzing salivary cortisol.