Postnatal weight gain in the first two weeks as a predicting factor of severe retinopathy of prematurity requiring treatment
Article information
Abstract
Purpose
This study aimed to investigate the relative weight gain at 2-week intervals up to 6 weeks after birth to predict retinopathy of prematurity (ROP) requiring treatment among very low birth weight infants.
Methods
A total of 211 preterm infants with birth weights <1,500 g and gestational age <32 weeks were retrospectively reviewed. The main outcome was the development of ROP requiring treatment. Body weight measurements were recorded daily. Relative weight gains (g/kg/day) were calculated at the second, fourth, and sixth week after birth.
Results
Of the 211 infants, 89 developed ROP, of which 41 spontaneously regressed and 48 with early treatment of ROP type I required laser treatment. The relative weight gain at 2, 4, and 6 weeks postnatal age was significantly lower in infants with ROP requiring treatment than in infants without ROP or those with spontaneous regression (P<0.001, P=0.005, and P=0.004, respectively). On logistic regression, poor relative weight gain in the first 2 weeks was found to be related to ROP requiring treatment (adjusted odds ratio, 0.809; 95% confidence interval, 0.695-0.941; P=0.006). Relative weight gain at 2 weeks postnatal age was significantly lower in infants with ROP requiring treatment compared to that in ROP requiring no treatment (P=0.012).
Conclusion
Poor postnatal weight gain in the first 2 weeks of life is an important and independent risk factor for ROP requiring treatment. Postnatal weight gain can predict the development of severe ROP requiring treatment.
Introduction
Retinopathy of prematurity (ROP), a complex disease of the developing retinal vasculature in premature infants, continues to be a major cause of childhood blindness in both developed and developing countries1,2). Most cases of ROP are mild or moderate that will regress spontaneously. However, severe ROP causes retinal detachment and permanent vision loss. Recently, improvements in perinatal services and neonatal outcome among preterm infants have led to an increased incidence of severe ROP3).
Multiple systemic risk factors are associated with the severity of ROP. The etiology and pathogenesis of ROP are not fully understood. ROP appears to develop in two phases. Initially, suppression of growth factors due to hyperoxia and the loss of maternalfetal interaction result in an arrest of retinal vascularization (phase 1). Subsequently, the increasing metabolically active, yet poorly vascularized, retina becomes hypoxic, stimulating growth factor-induced vasoproliferation (phase 2), which can cause retinal detachment4). The lack of insulin-like growth factor 1 (IGF-1), crucial for the normal growth and development of many tissues, including brain and blood vessels, that prevents normal retinal vasculature in phase 1 of ROP5). Early-postnatal low serum IGF-1 concentrations in preterm infants is strongly associated with later ROP5).
Current, screening guidelines for ROP use birth weight (BW) and gestational age (GA) to examine infants for treatable disease. These fixed prenatal factors cannot be modified to prevent the development of ROP. Therefore, the identification of modifiable postnatal risk factors that can be used to predict which infants have the potential to develop severe ROP requiring treatment would be beneficial. Postnatal weight gain has been suggested as one of the strongest predictors of ROP during the early weeks of life. However, it was unclear whether postnatal weight gain could be a predictor for severe ROP requiring treatment. Therefore, the objective of this study was to determine the relative weight gain in 2-week intervals up to 6 weeks after birth in order to predict the development of severe ROP requiring treatment among very low birth weight (VLBW) infants.
Materials and methods
We retrospectively evaluated the medical records of 211 preterm infants who were hospitalized for over 6 weeks in the neonatal intensive care unit of Soonchunhyang University Bucheon Hospital. Preterm infants born with BW<1,500 g and GA<32 weeks between January 2002 and December 2013 were recruited.
ROP was categorized according to the revised international classification of ROP6). Each child was classified according to the most advanced ROP stage observed. The screening examination for ROP followed the guidelines proposed by American Academy of Ophthalmology and Pediatrics and the Association for Pediatric Ophthalmology and Strabismus with some modifications7). The first screening examination was performed at 30- to 31-week postmenstrual age in infants born at <27 weeks GA and at 4- to 5-week postnatal age in infants born at 27- to 32-week GA. After the first evaluation, if the subject did not have ROP, he or she was evaluated at 2-week intervals until full vascularization. If the patient had active or rapid progressive lesion, the subject was evaluated in 2-day to 1-week intervals, depending on the clinical findings. Treatment criteria were based on the early treatment of ROP (ET-ROP) type 1 disease: included zone I, any stage of ROP with plus disease; zone I, stage 3 ROP with or without plus disease; and zone II, stage 2 or 3 ROP with plus dieases8,9). After reviewing the medical records, preterm infants were divided into the following three groups: (1) no ROP, (2) ROP requiring no treatment, and (3) ROP requiring treatment. We classified these three groups into two different models. In the first model, 211 of infants were divided into two groups: (1) as no ROP or ROP requiring no treatment, (2) ROP requiring treatment. In the second model, 89 of ROP infants were divided into ROP requiring no treatment and ROP requiring treatment.
We evaluated the following clinical parameters: GA (in weeks), BW (in grams), small for GA (BW <10th percentile), gender, type of delivery, multiple birth number, Apgar score at 1 minute and 5 minutes, maternal age, maternal history (preeclampsia, diabetes mellitus, oligohydramnios, premature rupture of membrane, intrauterine growth retardation), antenatal steroid treatment, hospital stay duration, mechanical ventilation, oxygen therapy, time to total enteral feeding, type of feeding, number of transfusions, and other morbidities (respiratory distress syndrome, bronchopulmonary dysplasia, patent ductus ateriosus, hypotension, necrotizing enteritis, grades III or IV intraventricular hemorrhage, periventricular leukomalacia, sepsis). Body weight measurements daily for the first six weeks of postmenstrual age, the days to regain BW, and the maximum weight loss percentage were recorded. Relative weight gains (body weight minus BW, divided by BW and postnatal age, g/kg/day) at the second, fourth, and sixth weeks of life were calculated.
All statistical analyses were conducted using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). Mann-Whitney U tests were used to analyze nonnormally distributed continuous variables. Chi-square test or Fisher exact test was used to compare categorical variables. Logistic regression was used to find to independent risk factors of ROP requiring treatment. All the risk factors found to be significant in univariate analysis without multicollinearity using a variation inflation factor (VIF) were included. Statistical significance was accepted at P<0.05. The results are presented as number (n), frequencies (%), and medians with their interquartile range (IQR). Receiver operating characteristic (ROC) curve was calculated to determine the best discriminative cutoff of relative weight gain to predict ROP requiring treatment.
Results
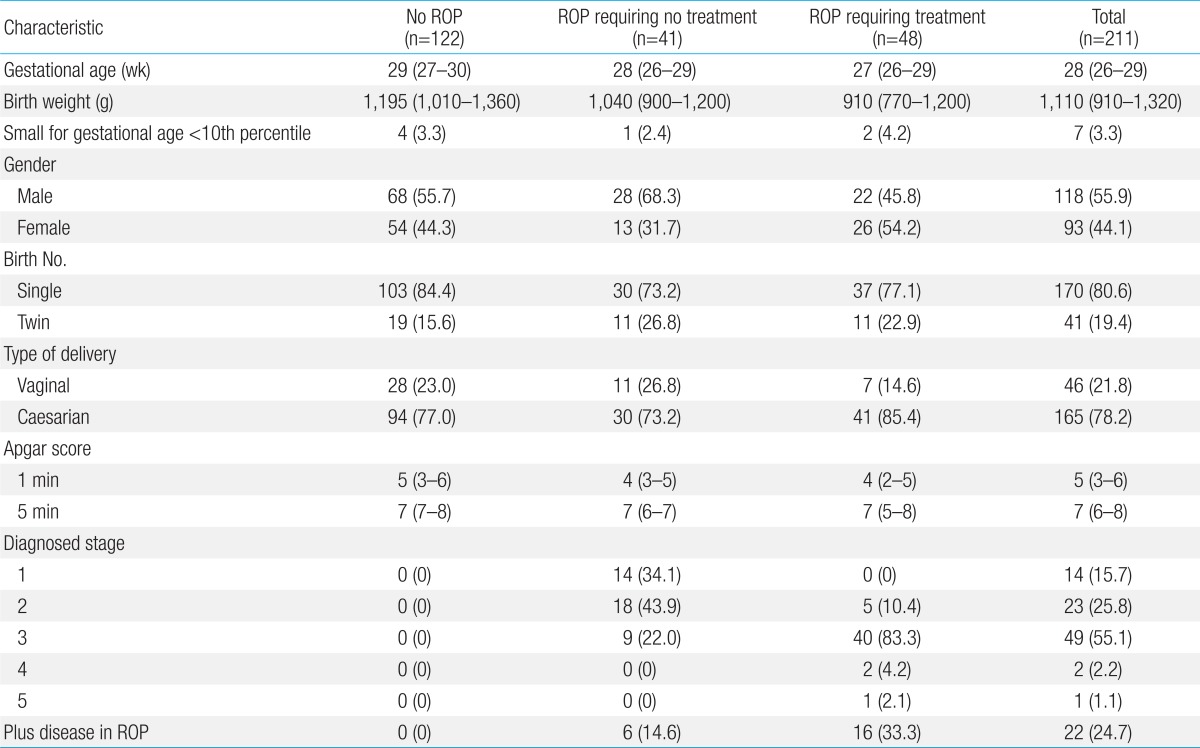
Demographics of the included infants are shown in Table 1. The median GA at birth was 28 weeks (IQR, 26-29) and the median BW was 1,110 g (IQR, 910-1,320). Male infants accounted for 55.9% of the study population (118 of 211); 170 infants (80.6%) were singleton births, 41 (19.4%) were twin births.
Of the 211 infants, 89 (42.2%) developed ROP, including 41 infants (46.1%) who developed ROP that spontaneously regressed and 48 ROP infants (53.9%) with ET-ROP type I for whom laser treatment was performed in both eyes.
In this study, two different models were used. In the first model, the study group included 48 preterm infants with ROP requiring treatment, and 163 preterm infants with no ROP or ROP requiring no treatment. Patients with ROP requiring treatment had significantly lower BWs (median, 910 g; IQR, 770-1,200; P<0.001), GA (median, 27 weeks; IQR, 26-29; P<0.001), and Apgar score at 1 minute and 5 minutes (P=0.006 and P=0.023, respectively) compared to those with no ROP or ROP requiring no treatment. The time to initial total enteral feeding, the final total enteral feeding, and the time to regain BW were longer in infants with ROP requiring treatment, than those in the other two groups (P=0.007, P=0.001, and P=0.001, respectively). The number of hospital days (P<0.001), ventilation days (P<0.001), oxygen supplement days (P<0.001), fraction of inspired oxygen (FiO2)>0.4 oxygen supplement days (P<0.001), transfusions (P<0.001), and weight loss percentage (P=0.001) were also significantly higher among patients with ROP requiring treatment. In addition, the appearance of bronchopulmonary dysplasia, periventricular leukomalacia, and sepsis were significantly more common in the infants requiring treatment for ROP (P=0.022, P=0.032, and P<0.001, respectively). The infants requiring treatment for ROP had significantly lower relative weight gain at the second, fourth, and sixth week of life (P<0.001, P=0.005, and P=0.004, respectively) (Table 2).

Univariate analysis of risk factors between no ROP/ROP requiring no treatment and ROP requiring treatment group
In the second model, the study group included 48 preterm infants with ROP requiring treatment, and 41 preterm infants with ROP requiring no treatment. In patients with ROP requiring treatment, male (P=0.033) and periventricular leukomalacia (P=0.047) were significantly more common and FiO2>0.4 oxygen supplement days (P<0.001) was significantly higher. The relative weight gain at the second weeks of life (P=0.012) was significantly lower in infants with ROP requiring treatment. However, there was no significant difference at the fourth or sixth weeks of life (Table 3).
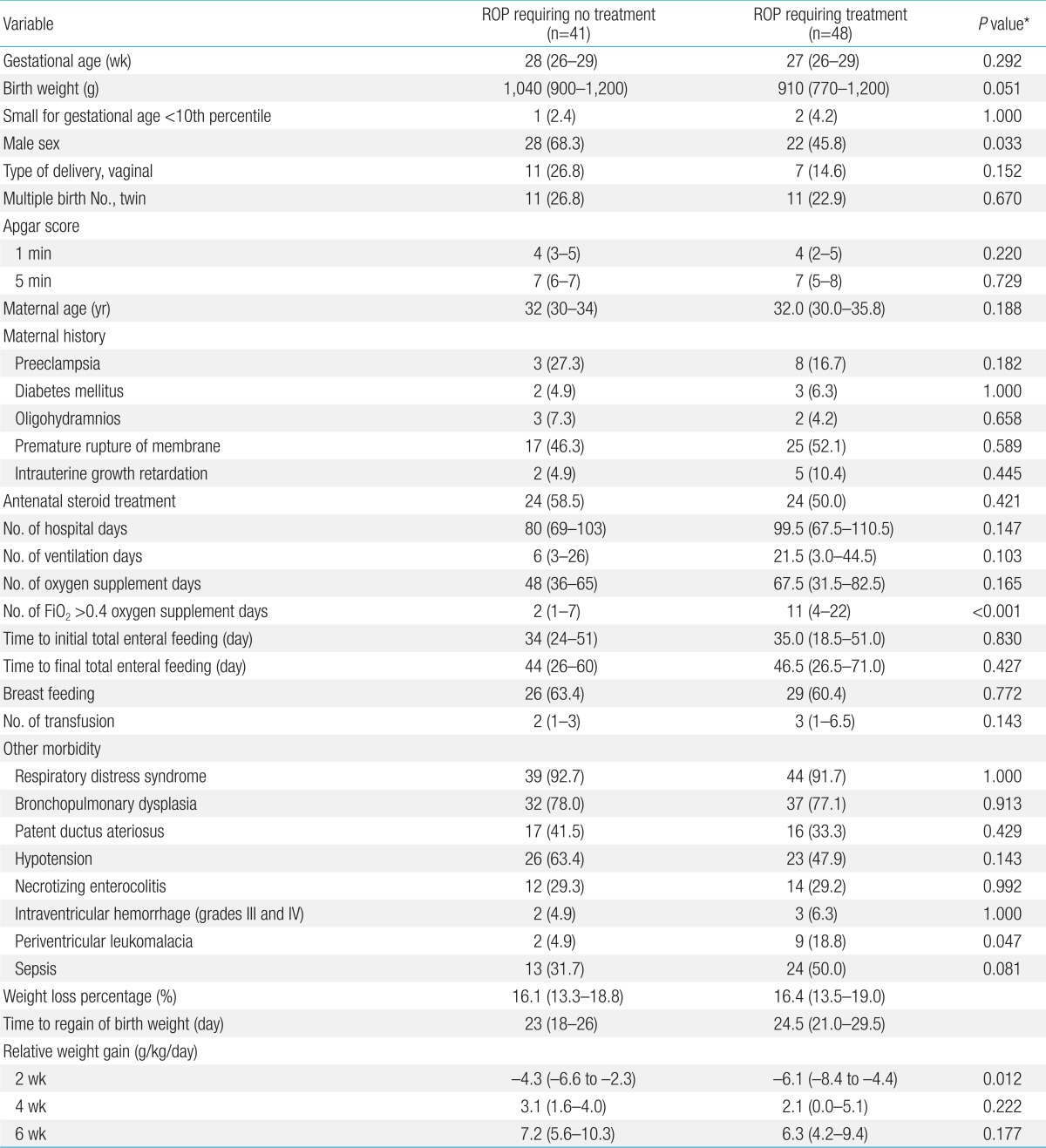
Univariate analysis of the risk factors of ROP requiring no treatment and ROP requiring treatment group
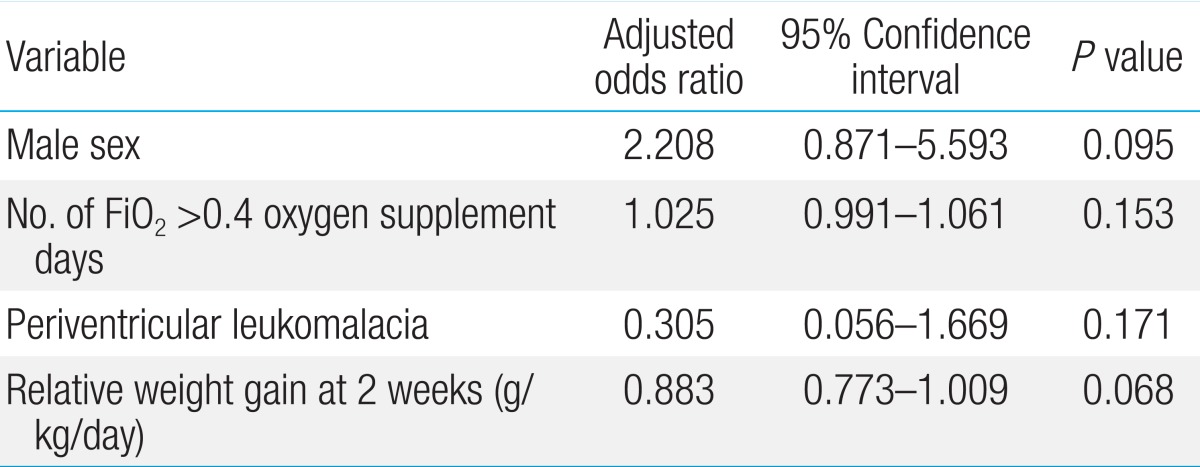
In the first model, all the risk factors found to be significant in univariate analysis were tested to determine multicollinearity (defined as a VIF>8) between each other. GA, BW, number of hospital days, ventilation days, FiO2>0.4 oxygen supplement days, transfusion, time to initial and final total enteral feedings, time to regain BW, bronchopulmonary dysplasia, periventricular leukomalacia, sepsis, and relative weight gain at second weeks of life were put into a logistic regression model. In this model, number of FiO2>0.4 oxygen supplement days (P=0.017) and relative weight gain at the second week of life (P=0.006) were found to be independently related to ROP treatment (Table 4).
Logistic regression analysis between ROP requiring treatment and no ROP/ROP requiring no treatment group
For ROP requiring treatment, ROC curve analysis revealed a cutoff of -4.5 g/kg/day of the relative weight gain at second week of life (area under the curve, 0.70; 95% confidence interval, 0.619-0.780) with sensitivity and specificity values of 75.0% and 62.5% respectively.
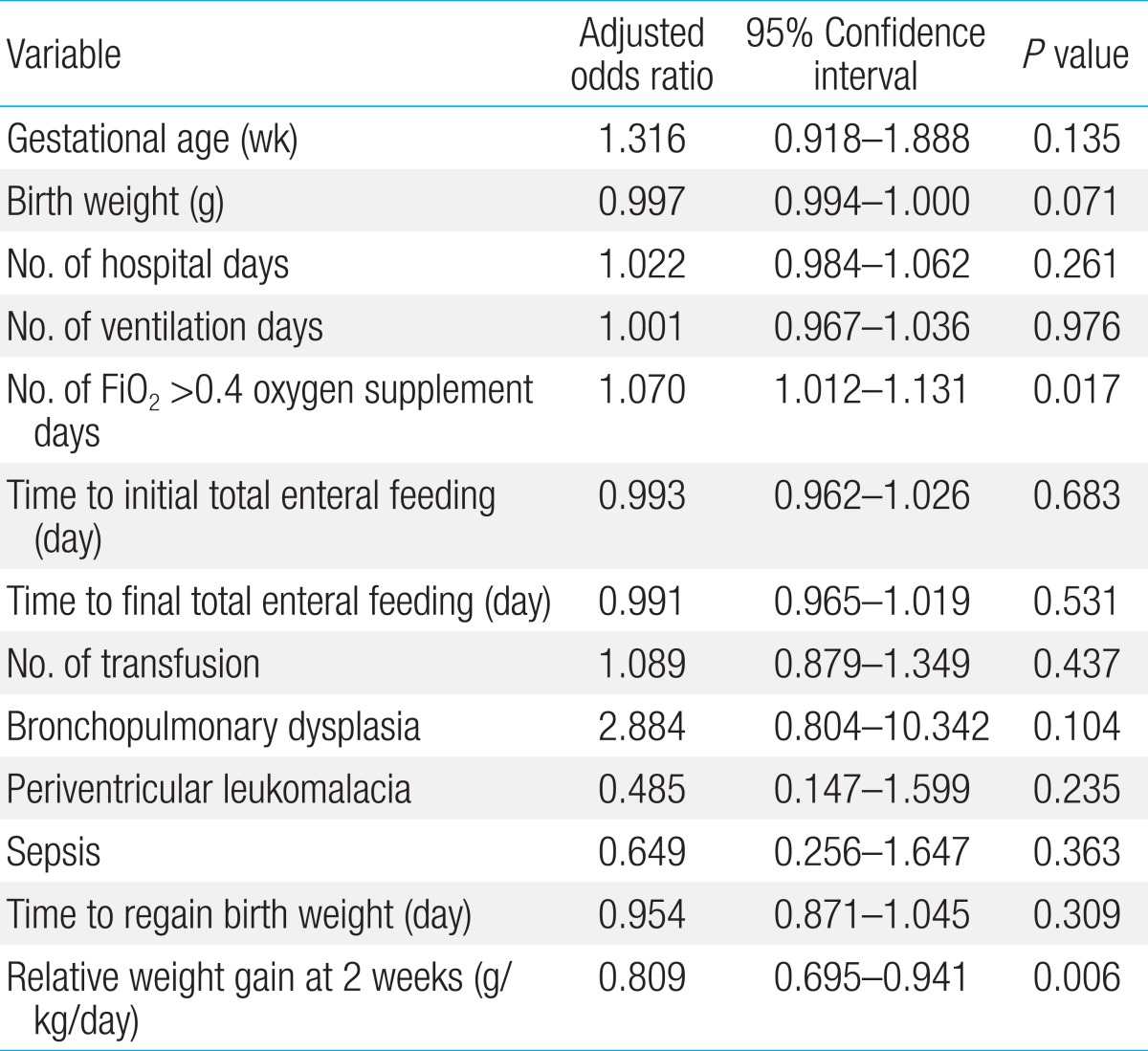
In the second model, male, FiO2>0.4 oxygen supplement days, periventricular leukomalacia, and relative weight gain at second weeks of life were put into a logistic regression model. All risk factor found to be significant in univariate analysis were not independent risk factor at this model (Table 5).
Discussion
Wallace et al.10) and Allegaert et al.11) reported an independent association between the absolute postnatal weight gain (g/day) at 6 weeks of life and severity of ROP, identified by logistic regression analysis. Fortes Filho et al.12) reported low weight gain proportion (body weight measured at 6 weeks of life minus BW, divided by BW) to be an important and independent risk factor for developing ROP which requires treatment. These studies were different from the present study in that they reported only weight gain at the sixth week of life, which was defined as absolute weight gain or weight gain proportion; however, these definition of weight gain have limitations for reflecting the true weight gain of preterm infants, because the preterm infants were all born with different BWs, and smaller preterm infants are expected to have lower weight gain compared to bigger infants. Therefore, relative weight gain (g/kg/day) is a more appropriate definition. Aydemir et al.13) used a similar method to that herein and found the second and fourth week of life to be significantly in univariate analysis, indicating relative weight gain in the fourth week of life was independently lower in infants with severe ROP.
Infants born preterm undergo weight loss and halting of reticular vascularization as consequence of loss of maternal-fetal interaction, different form the fetus in the uterus. In the aspect of maintaining weight gain at similar levels as in the fetus to lead to normal growth of retina vessels, preterm infants had are required to catch up with the fetus's weight gain in the early postnatal age. In first model of this study, relative weight gain at the second week of life was found to be an independent risk factor of ROP requiring treatment. This result may be regarded as providing emphasis on the importance of weight gain at an earlier postnatal age, compared with previous studies.
Poor weight gain in the early postnatal period could be associated with the development of general morbidities, some of which are also associated with the development of ROP, such as sepsis, intraventricular hemorrhage, bronchopulmonary dysplasia, and necrotizing enterocolitis. In the present study, infants with ROP requiring treatment had higher incidence of sepsis, bronchopulmonary dysplasia, and periventricular leukomalacia. In addition, infants born before GA 33 weeks had lower serum IGF-1 levels than full-term infants after birth. The low serum IGF-1 concentration will remain due to a very slow increase in IGF-1 production14,15,16). IGF-1 is essential for normal growth and the development of many tissues, including brain and blood vessels. Serum IGF-1 concentration is strongly association with postnatal weight gain of preterm infants and development of ROP4). Infants with poor weight gain in the early postnatal period could have high risk of developing ROP with subsequently requires treatment. This may explain why relative weight gain at the early postnatal age is the most important.
Our results revealed that the relative weight gain at the second week of life under -4.5 g/kg/day was an important and independent risk factor for ROP requiring treatment, and was capable of predicting the development of ROP requiring treatment in the group of VLBW infants.
In the second model, however, the relative weigh gain at the second week of life as independent risk factor of ROP requiring treatment did not arise as an independent risk factor of ROP requiring treatment. Kim et al.17) analyzed postnatal weight gain between ROP requiring nonsurgery and surgery. They reported that ROP requiring surgery was lower weight gain from the fourth to sixth week of life compared to ROP with non-surgery. Under a logistic regression analysis, an independent association between weight gain at eighth week of life and ROP requiring surgery was found. These differences with the present study might be due to strong association of poor weight gain with other unknown risk factors between ROP requiring treatment and not requiring treatment.
On logistic regression, FiO2>0.4 oxygen supplement days was a significant predictor for the development of ROP requiring treatment. Because oxygen tension is low in the uterus, preterm infants experience hyperoxia after birth. More importantly, supplemental oxygen given to preterm infants with respiratory distress can result in high oxygen saturation4). Hyperoxia interrupts normal retinal vascularization. This is followed by delayed retinal vascularization and then neovascularization4). Therefore, high concentrations of oxygen supplementation were considered the main risk factor for ROP development. Although no single study has conclusively determined the best method to predict the development of ROP. Strict management of oxygenation, minimalizing the alternation of hypoxia and hyperoxia while avoiding undesired high oxygen saturation during phase 1 seem to be the most promising strategy to prevent ROP.
Our study has some limitations. We assumed that low postnatal weight gain was related to low serum IGF-1 concentration. However, serum IGF-1 concentration was not measured in clinical practice. Therefore, we did not clearly show a relationship between them. We performed a retrospective study that extracted available data from medical records. The number of preterm infants in this study was relatively small. In addition, data were only from a single center that it could not reflect a population sufficiently. The intention of this study was to examine the association between the development of ROP requiring treatment and readily available clinical variables. Thus, no attempt was made to identify conditions that could affect early weight gain, such as parenteral nutrition.
In conclusion, the results of this study suggest that poor relative weight gain in the first 2 weeks of life is a predictor for severe ROP requiring treatment. It cannot be used as a screening tool alone, but it can help to identify infants with poor postnatal course who are at a greater risk. Thus, ophthalmologists and neonatologists should take special care and pay attention to this group of patients while screening for ROP.
Notes
No potential conflict of interest relevant to this article was reported.