Comprehensive understanding of atrial septal defects by imaging studies for successful transcatheter closure
Article information
Abstract
Transcatheter closure of atrial septal defects has become a popular procedure. The availability of a preprocedural imaging study is crucial for a safe and successful closure. Both the anatomy and morphology of the defect should be precisely evaluated before the procedure. Three-dimensional (3D) echocardiography and cardiac computed tomography are helpful for understanding the morphology of a defect, which is important because different defect morphologies could variously impact the results. During the procedure, real-time 3D echocardiography can be used to guide an accurate closure. The safety and efficiency of transcatheter closures of atrial septal defects could be improved through the use of detailed imaging studies.
Introduction
Atrial septal defect (ASD) is one of the most common congenital heart diseases encountered in outpatient clinics. Surgical closure has traditionally been accepted as the gold standard treatment for an ASD with volume overloading. Transcatheter device closure was developed as an alternative to surgery by King et al.1) in 1976 and accepted as a safe and effective treatment replacing surgical closure since the 2001 approval of the Amplatzer septal occluder (ASO) (St. Jude Medical Inc., St. Paul, MN, USA) by the United States Food and Drug Administration. However, due to the unfavorable anatomy of secundum ASD, a detailed evaluation of ASD has become crucial for device closure2,3). Thus, an evaluation of ASD in terms of successful transcatheter device closure is needed.
Diagnosis and indications for closure
Transthoracic echocardiography (TTE) is the gold standard for diagnosing ASD and has excellent sensitivity in young patients. In general, the subcostal view will definitely show ASD, and the apical four-chamber view and the parasternal short axis view are also helpful for making the diagnosis. However, when TTE imaging is poor due to obesity, previous thoracic surgery, or large breasts, transesophageal echocardiography (TEE) is an alternative that provides excellent images. High-resolution contrast computed tomography (CT) and advanced cardiac magnetic resonance imaging (MRI) can also be used to delineate ASD.
The general indications for closure include evidence of right cardiac volume overload even without symptoms. Before deciding on closure, bidirectional shunt and pulmonary hypertension associated with ASD should be evaluated with pulmonary vasodilators or the balloon occlusion test4). Indications for the closure of ASD in adults and children have been published by the American Heart Association5,6). The timing of the intervention should not be fixed. Most ASDs are asymptomatic, but symptomatic ASDs should be corrected surgically at any age. Elective surgical closure of asymptomatic ASDs is performed at 3-5 years of age7). Transcatheter closure of an ASD with the ASO has been recommended for patients weighing >15 kg. However, the weight restriction has eased over time, and many authors reported safe and effective device closure in patients weighing <15 kg8,9).
Anatomical evaluation
The sinus venosus, coronary sinus, and primum types of ASD require surgical closure while most secundum ASDs are amenable to transcatheter closure. In addition to the size of the ASD, the shape, number of defects, and surrounding rims are important for successful transcatheter closure. Usually, four or five rims should be evaluated before deciding on device closure. The anterior or retro-aortic rim can be evaluated on a parasternal short axis image. The posterior rim and anterior-inferior rim can be seen in the apical four-chamber view. The posterior-superior and posterior-inferior rims should be evaluated from the subcostal view or right parasternal view. In general, a sufficient septal rim should surround an ASD>5 mm in all directions10). Although retro-aortic rim deficiency is very common, most ASDs with deficient retro-aortic rims are closed successfully by the ASO11,12). However, an ASD with two or more deficient rims should not be closed with a device, as device embolization is highly expected in cases with significantly deficient posterior-inferior rims3). A sufficient rim is an indicator not only of length, but also of the floppiness of the rim tissue. Durongpisitkul et al.13) reported that a posterior-inferior rim length of at least 10 mm is safe for successful device closure of an ASD.
Two-dimensional (2D) echocardiography is an excellent method of evaluating ASDs. TTE has been used effectively and conveniently for ASD screening (Fig. 1), and TEE has been accepted as the best method for a complete evaluation of the defect for the purpose of device closure14) (Fig. 2). Nevertheless, the evaluation of the posterior-inferior rim may be incomplete in some patients13). TEE is uncomfortable for patients, and necessitates the use of sedation or anesthesia.
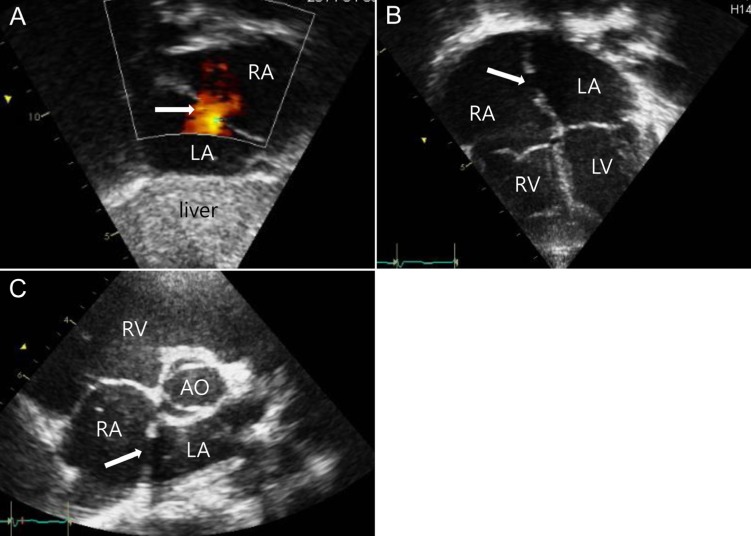
Transthoracic echocardiography (TTE) images of an atrial septal defect (ASD) in the subcostal view (A), four chamber view (B), and parasternal short axis view (C). The ASD indicated by a white arrow (A, B) and shunt flow demonstrated by the red color (A) were proven by TTE. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle; AO, aorta.
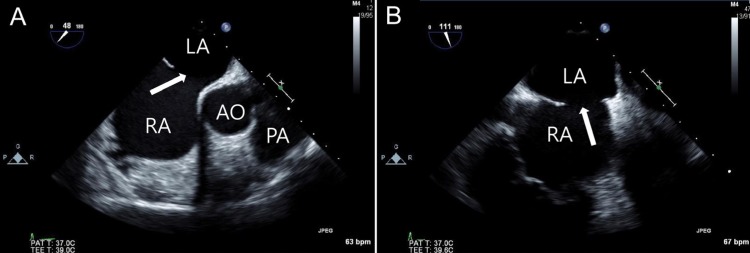
Transesophageal echocardiography images of an atrial septal defect (ASD) in a 48-degree view showing a 21-mm defect (A) and a 111-degree view showing an 18-mm defect (B). The white arrow indicates ASD. RA, right atrium; LA, left atrium; AO, aorta; PA, pulmonary artery.
By contrast, cardiac CT offers superb spatial and temporal resolution with a short scanning time. It allows for detailed imaging not only of intracardiac structures, but also of coexisting anomalies. In my experience, additive abnormal findings that include extracardiac lesions as well as cardiac lesions can be detected on CT images. My experience includes the detection of silent coronary stenosis and lung problems outside of the ASD on CT images. Some reports addressed the usefulness of cardiac CT for ASD device closure and demonstrated superior resolution and measurement of the surrounding rims using cardiac CT15,16) (Fig. 3). However, the radiation hazard posed by CT should not be ignored despite the use of a minimal exposure technique. We recommend cardiac CT for selected cases that have a complicated ASD or a large ASD with a rim deficiency.
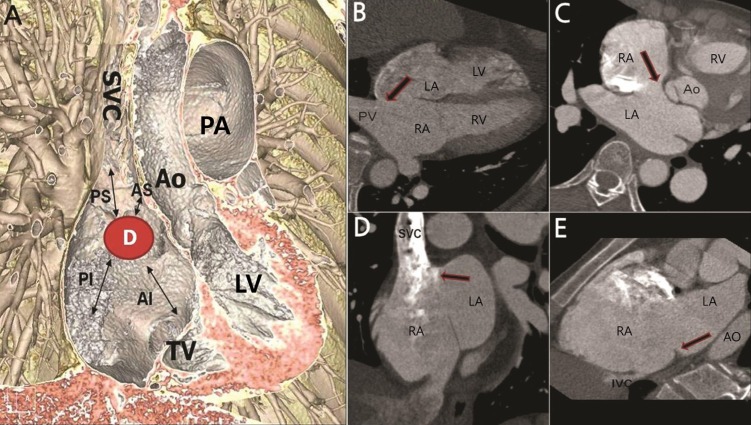
(A) A three-dimensional image showing four different rims surrounding the defect. (B) Arrow on the axial tomography image indicating posterior rim deficiency. (C) Arrow indicating anterior-superior rim deficiency. (D) Arrow indicating posterior-superior rim deficiency. (E) Arrow indicating posterior-inferior rim deficiency. D, atrial septal defect; AS, anterior-superior; AI, anterior-inferior; PS, posterior-superior; PI, posterior-inferior; SVC, superior vena cava; TV, tricuspid valve; LV, left ventricle; AO, aorta; PA, pulmonary artery; RA, right atrium; LA, left atrium; PV, pulmonary vein; RV, right ventricle; IVC, inferior vena cava.
Very few reports are available on the use of cardiac MRI for evaluating an ASD prior to device closure17,18). Although MRI poses no radiation hazard, the scanning time is much longer.
Shape and multiplicity
It is not surprising that many ASDs are not circular in shape, and indeed complex ASD shapes have been described by some authors16,19,20,21,22,23). Three-dimensional (3D) images should be reconstructed from 2D images for a complete understanding of defect shape. This can be achieved by real-time 3D TEE or 3D cardiac CT (Fig. 4). The preprocedural evaluation of an ASD undergoing device closure should be different from that used before surgical closure. Devices used for ASD closure do not reflect the various defect shapes, and all ASDs are assumed to be circular based on the longest diameter. However, the eccentricity of a defect could make a difference in device selection.
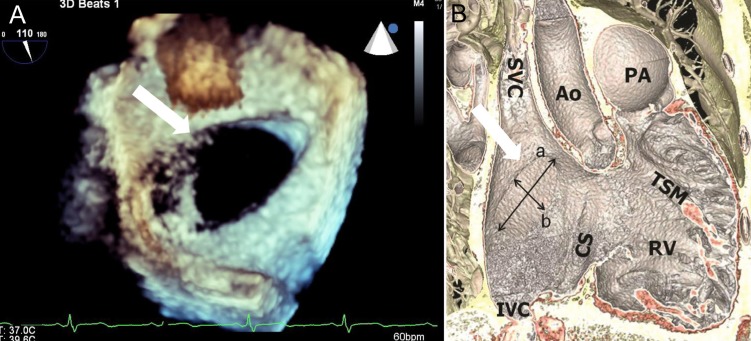
Three-dimensional reconstructed en-face images of an atrial septal defect (white arrow) showing an ovoid, as opposed to a circular, shape from transesophageal echocardiography (A) and cardiac computed tomography (B). a, longest diameter of defect; b, shortest diameter of defect; SVC, superior vena cava; IVC, inferior vena cava; CS, coronary sinus; AO, aorta; RV, right ventricle; TSM, trabecular septomarginalis; PA, pulmonary artery.
Device closure of an ASD that is 10 mm in the long diameter and 5 mm in the short diameter can differ from the closure of a nearly circular defect with a 10-mm diameter. Song et al.16) reported that an oval shaped ASD with a short diameter that was 75% smaller than the long diameter could be closed safely with an ASO smaller than that used for a circular defect. They also reported their experience of a successful transcatheter closure of an ASD that was 45 mm in the long diameter and 24 mm in the short diameter with a 38-mm ASO16).
Multiple ASDs should be evaluated precisely in terms of device closure (Fig. 5). It is well known that defects within 7 mm of each other can frequently be closed with a single device. We experienced the successful closure of multiple ASDs with multiple ASOs or a cribriform device24).
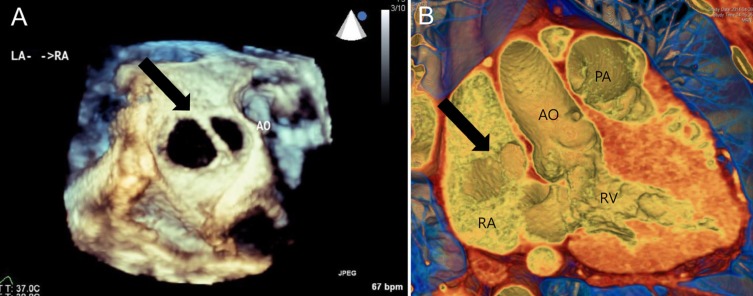
The same patient's three-dimensional reconstructed en-face images showing two atrial septal defects (black arrow) from transesophageal echocardiography (A) and cardiac computed tomography (B). AO, aorta; PA, pulmonary artery; RA, right atrium; LA, left atrium; RV, right ventricle.
Two-dimensional echocardiographic images have difficulty in comprehensively defining the shape of an ASD or multiple ASDs. By contrast, the excellence of 3D images has been reported by many interventionists. Reconstructed en-face CT or TEE images are superior for visualizing the morphology and the number of defects16,22,23,24,25). The morphology and location of the defect may be more easily understood on 3D images obtained from CT than those obtained from TEE. However, in the case of an ASD with a very thin or floppy interatrial septum, defect size and morphology can easily be distorted on the basis of CT images. Even though understanding anatomy and location based on TEE images can be complicated, they can provide a more accurate defect size and shape. At present, real-time 3D TEE is used widely due to the much shorter time required for 3D reconstruction.
Size evaluation
Balloon sizing has been accepted as an integral part of ASD device closure with an ASO, but several reports described techniques that did not use balloon sizing16,23,26,27). Oversizing has been suspected to play an important role in cardiac erosion after implantation and should be avoided during size selection. It is not surprising that a stretched balloon sizing technique results in frequent oversizing, which is why the stop-flow technique has replaced stretched balloon sizing28). Several reports have indicated that sizing with 3D TEE has similar results to those of the balloon sizing technique29,30). The longest diameter at the end-systole phase has been used to size the device, but defect morphology is often ignored when selecting device size. We identified a few reports that considered defect shape for deciding device size. Seo et al.31) found that devices implanted using the balloon sizing technique in large oval shaped defects were smaller than those used in large circular defects, and suggested that device size could be selected using 3D TEE linear parameters. By contrast, Song et al.23) measured defect area by planimetry on an en-face 3D TEE image and found that the defect area correlated nicely with the device size selected by the stop-flow technique (Fig. 6). Taken together, these data indicate that oversizing can be avoided by considering defect morphology in 3D images of the ASD.
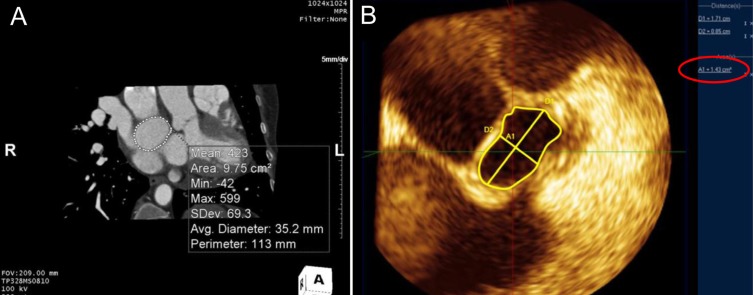
The defect area was measured by planimetry on en-face images from 3-dimentional (3D) computed tomography (A) and 3D transesophageal echocardiography (B). (A) The measured area was 9.75 cm2 and the average diameter was 35.2 mm. The longest diameter and shortest diameter of atrial septal defect were 17.1 mm and 8.5 mm, respectively. (B) The measured defect area described by the red circle was 1.41 cm2.
Guiding images
Echocardiography can be used for monitoring during ASD catheter closure. In most cases, 2D TEE or intracardiac echocardiography (ICE) has been used during the procedure even though TTE can be used in some cases. The efficacy and safety of ICE have been demonstrated32,33,34) (Fig. 7). Although it necessitates other venous access and a biplane image is not obtainable, ICE can be conveniently used by the same operator simultaneously. In particular, ICE provides a more accurate image of the posterior-inferior rim that is superior to a TEE image. An accurate size evaluation for adequate device size selection during the procedure can be performed by ICE. Rigatelli et al.35) reported their experience with ICE during ASD device closure, achieving a good outcome even without the use of balloon sizing methods.
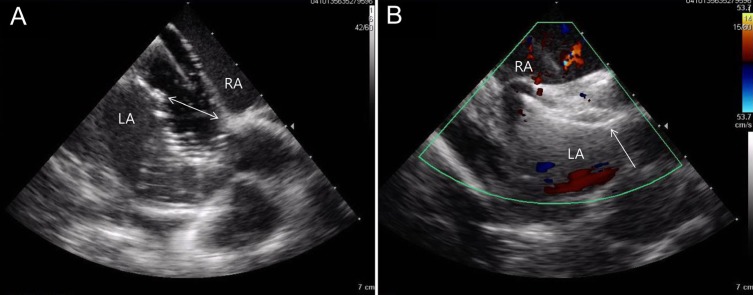
Images of balloon sizing (A) and successful device implantation (B) from intracardiac echocardiography during transcatheter closure of an atrial septal defect (ASD). The balloon occlusive diameter (two-way arrow) was 14 mm (A) and a 16-mm Amplatzer septal occluder (arrow) was positioned appropriately on the ASD (B). LA, left atrium; RA, right atrium.
Real-time 3D TEE provides several benefits during ASD device closure, such as visualization of the catheter and wire course, position of the device, and the interaction between the device and surrounding structures after implantation36). In the case of multiple ASDs or a multifenestrated ASD, wire selection should be monitored for successful complete closure, and real-time 3D TEE is very helpful37).
Conclusions
Although transcatheter closure of an ASD is technically simple and straightforward, an imaging study is potentially very important for the successful completion of the procedure. Three-dimensional TEE or CT reconstructed images are helpful for an accurate understanding of the morphology and preprocedure sizing. In cases of multiple ASDs, real-time 3D TEE can provide guidance during the procedure.
Notes
No potential conflict of interest relevant to this article was reported.