Two adolescent patients with coexistent Graves' disease and Moyamoya disease in Korea
Article information
Abstract
Moyamoya disease is a cerebrovascular condition that results in the narrowing of the vessels of the circle of Willis and collateral vessel formation at the base of the brain. Although relationships between Graves' disease and cerebrovascular accidents in Moyamoya disease are obscure, the coexistence of the two diseases is noteworthy. Moyamoya disease has been rarely reported in adolescent patients with thyrotoxicosis. Recently, we encountered two adolescent Korean patients with Moyamoya disease associated with Graves' disease who presented with episodic right-sided hemiparesis and syncope. These two girls who had Graves' disease had no history of other diseases or head trauma. A thyroid function test revealed a euthyroid state and a high thyroid-stimulating hormone (TSH) receptor antibody titer at that time. The patients were diagnosed with Moyamoya disease based on brain magnetic resonance angiography and cerebral four-vessel angiography. The patients underwent cranial revascularization by encephalo-duroarterio-synangiosis as soon as a diagnosis was made, which resulted in successful symptom resolution. They fared well and had no additional neurological symptoms as of their last follow-up visits. Here, we report these two cases of confirmed Moyamoya disease complicated by Graves' disease with a review of the literature, and discuss the possible association between the two diseases. To our knowledge, this is the first report in South Korea on Moyamoya disease associated with Graves' disease in adolescents with a euthyroid.
Introduction
Moyamoya disease is a disorder characterized by bilateral stenosis or occlusion of the terminal internal carotid artery and proximal portion of the anterior and middle cerebral arteries1). The pathogenesis of Moyamoya disease is unknown, but hyperactivity of cervical sympathetic nerves may contribute to stenosis of the cerebral arteries2). This disease has been reported in association with various disease entities, including hypothalamic-pituitary dysfunction, systemic lupus erythematosus, and Down syndrome3,4). Graves' disease is an autoimmune antibody-mediated disease of the thyroid gland that can lead to the development of Moyamoya disease4). Many symptoms of thyrotoxicosis are similar to those of hyperactivity of the sympathetic nervous system, and thyroid hormones are thought to enhance the effects of sympathetic nervous activity5). However, coexistence of thyrotoxicosis and Moyamoya disease is extremely rare6). Until now, Moyamoya disease has been rarely reported in adolescent patients with thyrotoxicosis in Korea7,8). We report two girls with concurrent Moyamoya disease and Graves' disease who presented episodes of syncope and right-sided hemiparesis.
Case reports
Case 1
A 12-year-old girl experienced a one minute episode of syncope with hyperventilation. She was diagnosed with Graves' disease 10 months ago, taking high dose of maintenance methimazole 20 mg daily and followed up regularly at the pediatric outpatient clinic of Dong-A University Hospital. She had no past history of other disease or head trauma. She showed no specific neurologic sign on physical examination at that time, either.
On admission, laboratory investigations, including routine complete blood count (CBC), erythrocyte sedimentation rat (ESR), biochemistry, C3, C4, fibrinogen, antithrombin III, protein C and S, homocysteine, cryoglobulin, antinuclear antibody (ANA), antineutrophil cytoplasmic antibody (ANCA), anti-DNA, anticardiolipine antibodies, and antiphospholipid antibodies, were all within normal ranges. Her serum level of triiodothyronine (T3), free thyroxine (FT4), thyroid stimulating hormone (TSH), and TSH receptor antibody was 213.36 ng/dL (reference range, 87-185 ng/dL), 1.64 ng/dL (reference range, 0.8-2.2 ng/dL), 0.24 uIU/mL (reference range, 0.3-4 uIU/mL), 112.66 U/L (reference range. <14.0 U/L), respectively. The patient was diagnosed with Moyamoya disease with typical stenosis of the right middle cerebral artery (MCA) based on brain magnetic resonance (MR) angiography (Fig. 1) and cerebral 4-vessel angiography (Fig. 2). Single-photon emission computed tomographic (SPECT) scans revealed cerebral hypoperfusion of the right parietal lobe and decreased vascular reserves of both occipital lobes (Fig. 3). The patient underwent cranial revascularization by encephaloduroarterio-synangiosis (EDAS). To date, she has not presented a syncope attack since the surgery.
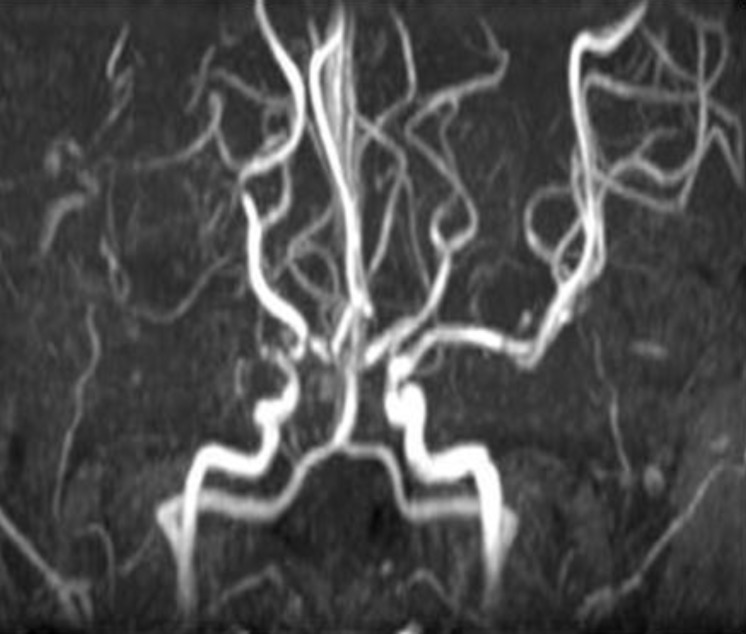
Brain magnetic resonance angiography revealing occlusion of the right middle cerebral artery and narrowing of the left distal internal carotid artery.
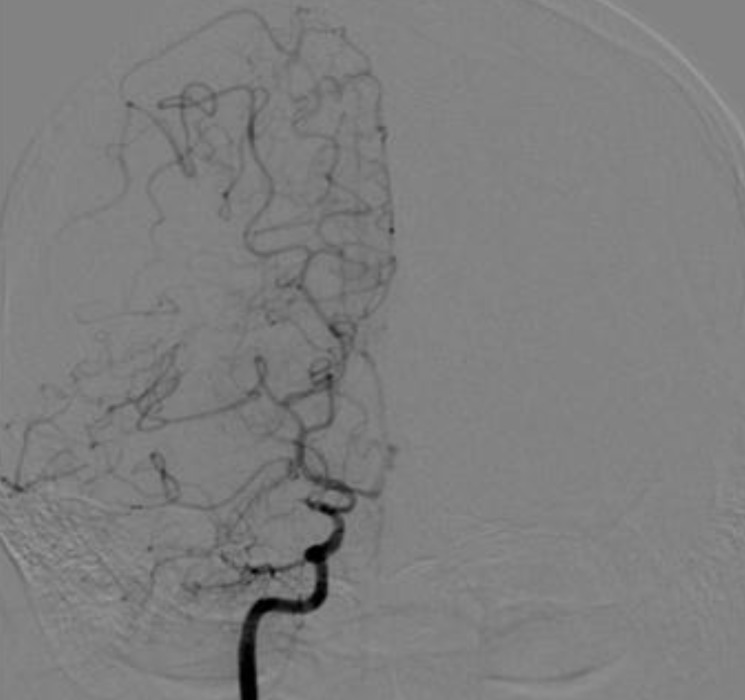
Examination by cerebral four-vessel angiography revealing nearly total occlusion of the right internal carotid artery bifurcation and a moderate degree of pial collateral flow from the right anterior and posterior cerebral artery to the middle cerebral artery territory.
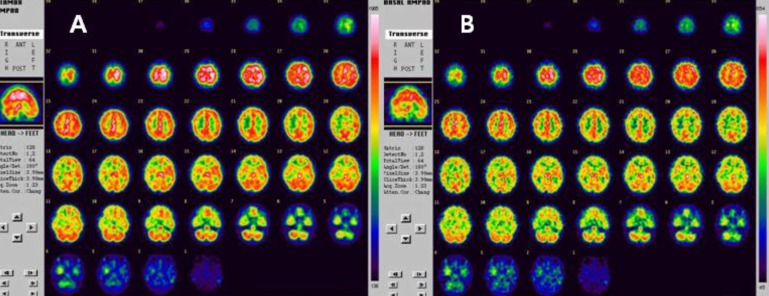
Technetium 99m-labeled hexamethylpropylene amine oxime showed decreased perfusion in the right parietal and both occipital lobes on basal single-photon emission computed tomography (SPECT) (A) and a decreased vascular reserve in the right frontal and temporal lobes, and partially preserved vascular reserve in the right parietal, both occipital, and left parietal lobes in postacetazolamide (Diamox) SPECT (B).
Case 2
A 15-year-old girl who had suffered from Graves' disease for seven years visited the Pediatric Endocrinology Clinic at Pusan National University Children's Hospital with episodic transient right-sided hemiparesis, along with the headache of 3 days' duration. She was being treated with 10 mg of methimazole daily. On physical examination, she had goiter and central type sided facial palsy.
On admission, laboratory investigations, including routine CBC, ESR, biochemistry, C3, C4, fibrinogen, antithrombin III, factor VIII, factor V, protein C and S, homocysteine, cryoglobulin, ANA, ANCA, anti-DNA, anticardiolipine antibodies, and antiphospholipid antibodies, were all within normal ranges. Her serum level of T3, FT4, TSH, and TSH receptor antibody was 1.07 ng/mL (reference range, 0.58-1.59 ng/mL), 0.99 ng/dL (reference range, 0.7-1.48 ng/dL), 0.01 uIU/mL (reference range, 0.27-4.20 uIU/mL), 34.1 U/mL (reference range, <9.0 U/mL), respectively.
Brain MR imaging scans showed focal hemorrhage at the left insula, basal ganglia, and focal enhancing nodule, with small collateral vessels in basal ganglia and obliteration of both internal carotid artery (ICA) (Fig. 4). The patient was diagnosed with Moyamoya disease with typical stenosis of the ICA and MCA, based on cerebral angiography (Fig. 5). SPECT scans revealed cerebral hypoperfusion of the left parietal lobe, left thalamus, left basal ganglia, and medial temporal lobe without vascular reserve (Fig. 6). The patient underwent cranial revascularization by EDAS. After surgery, the patient's symptoms improved and she recovered power to her right upper and lower extremities. She fared well and had no additional neurological symptoms as of her last follow-up visit.
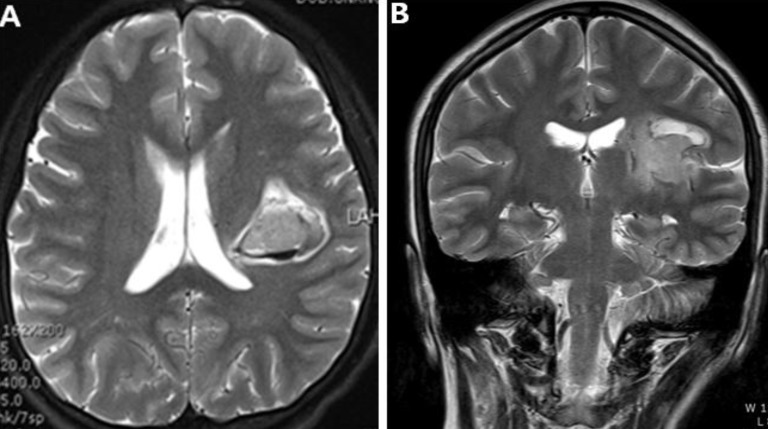
Brain magnetic resonance imaging scans showing focal hemorrhage at the left insula and basal ganglia (A) and a focal enhancing nodule within the small collateral vessels in the basal ganglia and obliteration of both internal carotid arteries (supraclinoid portion) on coronal T2-weighted imaging (B).
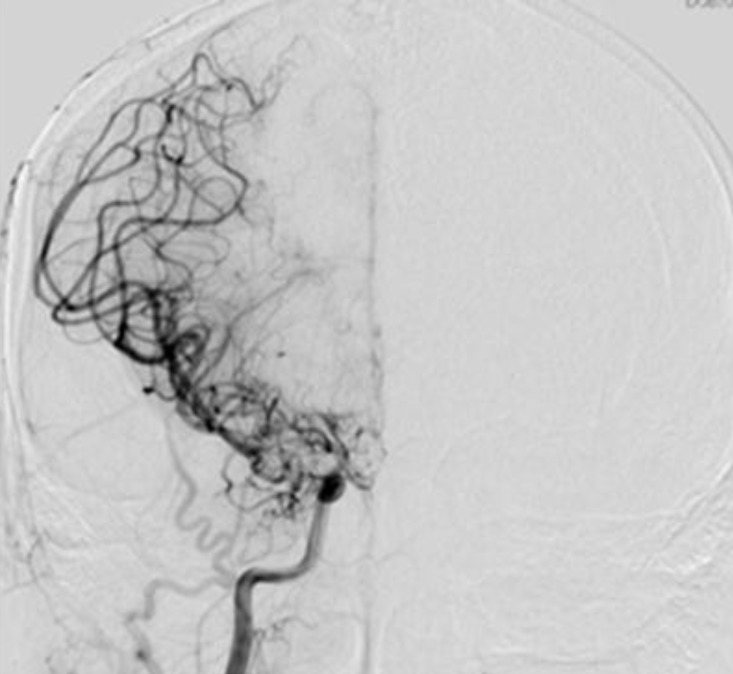
Four-vessel angiography demonstrating narrowing of both the M1 and distal internal carotid artery (ICA) with a fine distal ICA with a fine Moyamoya vessel.
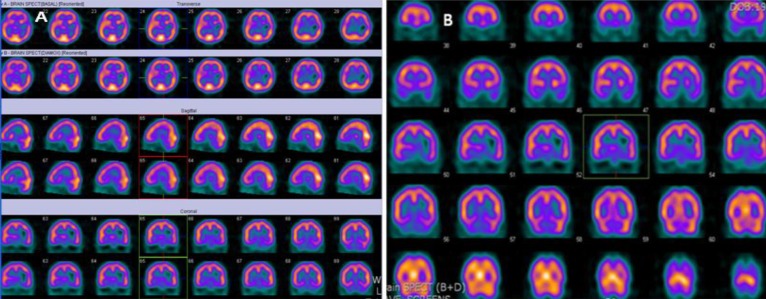
Technetium 99m-ethyl cysteinate dimer showing decreased perfusion in the left parietal lobe, left thalamus, left basal ganglia, and medial temporal lobe in brain single-photon emission computed tomography (SPECT) (A) and decreased vascular reserve in the temporal lobe and no vascular reserve in left basal ganglia in postacetazolamide (Diamox) SPECT (B).
Discussion
Moyamoya disease is a cerebrovascular condition that results in the narrowing of the vessels of the circle of Willis and collateral vessel formation at the base of the brain2,8). It becomes symptomatic whenever the collateral vessels fail to compensate for the cerebral blood flow deficit produced by the progressive stenotic lesion in the major cerebral arteries5). Although relationships between Graves' disease and cerebrovascular accidents in Moyamoya disease are obscure, the coexistence of both diseases is rare and noteworthy. To our knowledge, only 33 cases, including our case, of concurrent Moyamoya disease or intracranial arterial stenosis/occlusion and Graves' disease have been described in the English literature1,2,4,5). The interrelationship of these disorders is significant because the common pathogenesis to the two diseases may finally help us to elucidate the etiology of Moyamoya disease.
Cerebrovascular ischemic symptoms in Moyamoya disease are usually precipitated by activities associated with hyperventilation or thyrotoxicosis2,8). In our case 1, the cerebrovascular ischemic symptom, syncope was aggravated by hyperventilation regardless of a state of euthyroid. Hyperventilation itself might cause cerebral vasoconstriction resulted in the decrement of cerebral blood flow to ischemic levels. Whereas it is believed that excess thyroid hormone in patients with Graves' disease is harmful to arterial walls because it may alter vascular reactivity and accelerate the formation of abnormal cerebral vessels7). In the majority of cases, cerebrovascular accidents occurred when patients were thyrotoxic, suggesting that thyroid hormones could have facilitated these accidents, possibly through hemodynamic changes and (or) sympathetic nervous system-mediatedvasculopathy9,10). Sympathetic nervous tone itself is not accentuated by thyrotoxicosis, but thyroid hormones are thought to increase sensitivity to the sympathetic nervous system4). Other disorders associated with Moyamoya disease have well-established autoimmune mechanisms of pathogenesis or are associated with arteritis that affects the cerebral arteries7). Such diseases include systemic lupus erythematosus, antiphospholipid syndrome, ulcerative colitis, tuberculosis, leptospirosis and postradiation arteritis7). In clinical practice, the value of measuring sedimentation rate, protein S and C, antithrombin III, anticardiolipin antibodies, antinuclear factor, anti-Ro/SS-A, and anti-La/SS-B antibodies remains to be determined11). Another possible link between these disorders is atherosclerosis. Colleran et al.12) demonstrated the positive correlation between free thyroxine levels and both homocysteine and methylmalonic acid, suggesting the possibility of thyrotoxicosis inducing hyperhomocysteinemia. As homocysteinemia has been implicated in atherosclerotic and embolic disorders, it would be another key to the pathophysiology of Moyamoya disease12). It is practical to consider patients with both multiple arterial stenosis and Graves' disease as being at risk for ischemic cerebrovascular events. Therefore, careful control of thyroid function is essential.
A recently published study observed that patients with Moyamoya disease were more likely to have elevated thyroid auto-antibodies than those with non-Moyamoya disease strokes, despite being in a euthyroid state13). Two patients in this study also had high levels TSH receptor antibody when syncope and right-side hemiparesis developed, even in a euthyroid state. This suggests a possible underlying immune process contributing to the development of Moyamoya disease4). In addition, there are familial cases of Moyamoya disease and Graves' disease, indicating hereditary involvement in both diseases14). Taken together, genetic factors and autoimmunity may play important roles in the pathogenesis of the coexistence of Moyamoya disease and Graves' disease6).
On the other hand, therapy with steroids, vasodilators, antibiotics, and nerve blocks appear to be of questionable benefit. Miyamoto et al.15) observed that superficial temporal artery-middle cerebral artery anastomosis with encephalomyosynangiosis was an appropriate surgical treatment for refractory cases of Moyamoya disease, and surgical revascularization may be considered for select patients. Adequate revascularization was confirmed on both cerebral angiography and SPECT studies, which were performed 3 to 6 months after surgery15). Our patients underwent surgery as soon as a diagnosis was made, which resulted in successful resolutions of symptoms.
In conclusion, this is the first report of Moyamoya disease associated with Graves' disease in two adolescent patients with euthyroid in Korea. We suggest that Moyamoya disease should be considered when patients with Graves' disease, present with central nervous system symptoms, including headache, hemiparesis, and syncope. Further studies for large-scale national and multi-institutional patients are warranted to better understand the probable common underlying mechanisms of Moyamoya disease and Graves' disease.
Acknowledgment
We thank the patients and their families for participating in this study, which was supported by a 2-Year Research Grant of Pusan National University.
Notes
No potential conflict of interest relevant to this article was reported