Moyamoya syndrome occurred in a girl with an inactive systemic lupus erythematosus
Article information
Abstract
We report the case of a 17-year-old Korean girl with systemic lupus erythematosus (SLE) who presented with sudden weakness of the right-sided extremities and dysarthria. Oral prednisolone was being taken to control SLE. Results of clinical and laboratory examinations did not show any evidence of antiphospholipid syndrome or thromboembolic disease nor SLE activity. Cerebral angiography showed stenosis of the left internal carotid artery and right anterior cerebral artery with accompanying collateral circulation (moyamoya vessels). After the patient underwent bypass surgery on the left side, she recovered from the neurological problems and did not experience any additional ischemic attack during the 14-month follow-up period. This case represents an unusual association between moyamoya syndrome and inactive SLE (inactive for a relatively long interval of 2 years) in a young girl.
Introduction
Moyamoya disease (MMD) is a cerebrovascular disorder of unknown etiology that is recognized by bilateral progressive stenosis or occlusion of the terminal portions of the internal carotid artery (ICA) and the development of an abnormal vascular network or moyamoya vessels (MMVs) around the arterial occlusion1). Moyamoya syndrome (MMS) is a term applied to describe the MMVs associated with underlying causes such as vasculitis, fibromuscular dysphasia, sickle cell disease, or others. Several reports have described the association between MMS and autoimmune diseases2-5).
Only a few reports have linked MMS with systemic lupus erythematosus (SLE) (Table 1)2,5-8). A Korean girl presented symptoms of severe headache within one year after diagnosis of SLE diagnoses, which was then diagnosed as a familial MMS associated with SLE5). Another 18-year-old Korean woman exhibited MMS associated with the aggravated active lupus nephritis within the first week of SLE diagnosis6). A 20-year-old Chinese woman showed sudden right hemiplegia, and was disagnosed as bilateral MMS associated with deteriorating SLE, whose symptoms of SLE started at the age of 10 years8).
In this report, we present an unusual case of patient who presented sudden weakness of right-sided extremities and dysarthria without other associated SLE disease activity at 2 years after SLE onset, and who completely recovered without further ischemic attack through bypass surgery. We also present a literature review of the few reported MMS cases associated with SLE.
Case report
A 17-year-old Korean female was brought to the pediatric department because of new-onset, right-sided weakness that was first noted 3 weeks ago when it affected her writing ability. Her speech was slurred and she complained tongue numbness. Her symptoms seemed to improve for a while at first, but afterward she did not completely recover for 1 month. There was no evidence of trauma, flu-like symptoms, or no report of nausea, vomiting, headache, or convulsions. Evaluating the patient's medical history, there were no complications at birth, and her development had been age appropriate. There was no family history of stroke in young individuals.
In September 2008, at 15 years of age, she was diagnosed with lupus nephritis. Initial clinical course showed severe manifestations with microangiopathic hemolytic anemia, thrombocytopenia, and hypertension. The renal biopsy finding indicated the lupus nephritis ISN/RPS Class IV-G(A). After the initial period of 6 months, her clinical symptoms were well controlled with treatment of azathioprine, prednisolone and antihypertensive drugs. However, after 22 months, new-onset right-sided weakness developed.
On admission, vital signs were stable. Neurological examination revealed asymmetric motor weakness with the grade of 4/5 in the right arm and leg. She was mentally alert, but had a subtle dysarthric speech despite of normal cranial nerves. Reflexes for both knees were symmetric and normal. Otherwise, her systemic examination was unremarkable.
The laboratory tests revealed the followings: leukocyte, 5,100/mm3; hemoglobin, 14.8 g/dL; platelet, 266,000/mm3; erythrocyte sedimentation rate, 2 mm/hr (0-20); C-reactive protein level, 0.01 mg/dL (0-0.5); C3, 64 mg/dL (79-152); C4, 12.4 mg/dL (10-40); and CH50, 37.6 U/mL (23-46). Electrolytes, renal and hepatic profiles, and lipid profile were normal. Anti-thrombin III, prothrombin time, activated partial thromboplastin time, protein C (120%), protein S (70%), and lipid profiles were all within normal limits. Urine analysis showed no proteinuria or microscopic hematuria. Immunologic evaluation revealed antinuclear antibody (ANA) of 1:80, antids-DNA immunoglobulin G (IgG)/IgM 7 (0.25)/14 (0.20) IU/mL, anticardiolipin IgG/IgM (-/-), lupus anticoagulant (-), antiphospholipid antibody (-), anti-Ro/anti-La (-/-), and venereal disease research laboratory test (VDRL) (-). Chest X-ray, electrocardiogram, and echocardiogram findings were all unremarkable. There was no evidence of aggravation of SLE activity.
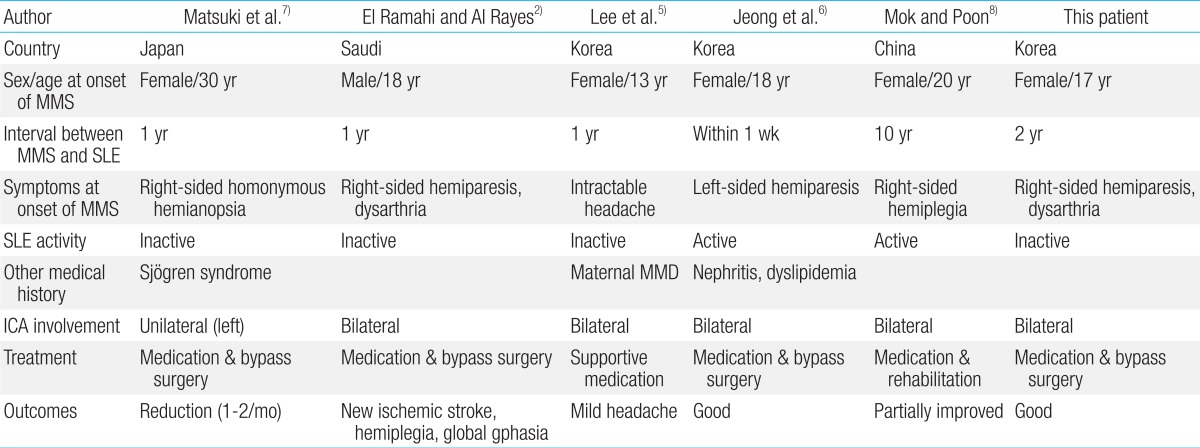
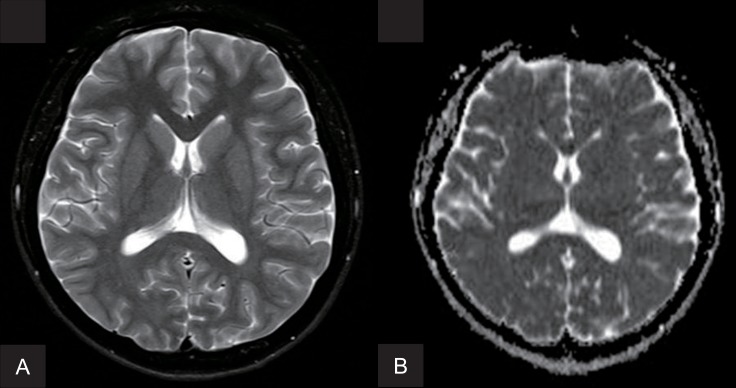
Brain magnetic resonance imaging (MRI) revealed no evidence of cerebral infarction or ischemic change (Fig. 1). Magnetic resonance angiography of the brain revealed stenosis of the supraclinoid portion of the left ICA and the right anterior cerebral artery (ACA). Conventional angiography showed severe narrowing of the distal portion of left ICA, the proximal portion of left middle cerebral artery (MCA), ACA and the right ACA (Fig. 2). These findings were compatible with MMD.
Brain magnetic resonance imaging (MRI). Findings of axial T2-weighted MRI (A) and diffusion-weighted MRI (B) at the basal ganglia level are unremarkable, with no evidence of acute infarctions.
BConventional cerebral angiograms of both carotid arteries. (A) Left internal carotid arteriogram shows diffuse stenosis of the supraclinoid portion of the left internal carotid artery (arrowhead) and the proximal portion of the left middle cerebral artery (arrow) with (B) collateral circulation from the left posterior cerebral artery (arrowheads). (C, D) Right internal carotid arteriogram shows severe stenosis of the A1 portion of the right anterior cerebral artery (arrowheads).
The patient was started aspirin, however, her right-sided weakness and dysarthria continued unabated. One week later, left encephalo-duro-arterio-synangiosis bypass surgery was performed. Postoperative MRI showed no interval change of right MCA branch and no further progression with new lesion. She has since recovered without new ischemic symptoms. No further neurological deficits occurred during a 14-month follow-up. Right-side surgery will be scheduled depending on follow-up neuroimaging results and the patient's neurological symptoms.
Discussion
Our patient was a remarkable case of MMS in a 17-year-old girl with SLE. The right-side hemiparesis developed at 2 years after SLE onset without SLE disease activity. The incidence of cerebrovascular accidents (CVA) in SLE cases is 2% to 12% within the first several years9,10). Symptomatic occlusion or stenosis of large cerebral vessels in SLE usually occurs within several years of SLE onset during the active period of the illness. However, cerebrovasculopathy in SLE may occur even without vigorous disease activity11).
There have been few reported MMS cases associated with SLE (Table 1)2,5-8). Matsuki et al.7), reported the first case of SLE and Sjogren syndrome associated with unilateral MMVs in cerebral arteries in the absence of antiphospholipid syndrome. The patient was a 30-year-old Japanese woman who presented with recurrent, right-sided homonymous hemianopsia without major organ involvement of SLE.
An 18-year-old Saudi male with inactive SLE presented right-sided hemiparesis one year after SLE onset without any accompanying risk factors for CVA2). Angiography revealed bilateral ICA stenosis with MMVs. This patient was the first documented case of SLE associated "bilateral" MMVs. He was medically treated for 3 weeks before the decision to perform bypass surgery. Despite the surgery, he developed new ischemic stroke and multiple cranial nerve palsies. Reoperation and medical treatment was performed, and no additional attacks occurred during a 9-month follow-up period.
Lee et al5), described a 13-year-old Korean girl with SLE who developed severe migraine-type headaches one year after onset. Patient's mother had been diagnosed with MMD. There was no symptomatic evidence of SLE disease activity or other risk factors of CVA. However, her magnetic resonance angiography revealed bilateral ICA stenosis with collateral circulation. Headache can be important indicator of MMD in SLE, occurring in a significant numbers of patients12).
Another 18-year-old Korean woman had sudden onset of left hemiparesis while being treated for active lupus nephritis within the first week of SLE diagnosis6). She did not have any history of seizure, or cardiovascular disorder. Brain MRI and angiography revealed multiple cerebral infarctions, and bilateral stenosis of the ICA was observed with abundant MMVs, which were consistent with MMS diagnosis.
A 20-year-old Chinese woman with SLE had sudden right hemiplegia, whose illness started at the age of 10 years8). She showed some evidence of active SLE as lupus flare with arthritis, proteinuria and deteriorating serology. Conventional angiography revealed bilateral occlusion of the supraophthalmic segments of the ICAs with extensive collateral MMVs. She was treated with pulse methylprednisolone, anticoagulation and rehabilitation for 3 months, and is scheduled for pial synangiosis. Among the previous reported five cases2,5-8), this Chinese woman showed the longest duration of the interval between initial SLE onset and subsequent diagnosis of MMS, which ranged from 1 week to 10 years.
The mechanism for manifestation of MMS following autoimmune disease remains unclear. However, MMS with positive indicators for ANA, anti-ds-DNA, anti-Ro, or lupus anticoagulant, may suggest the immunological role in MMS pathogenesis13). Jeong et al.6), hypothesized that underlying cerebrovascular lesions, such as MMVs with coexisting high lupus activity, would become more susceptible to CVA. In addition, hemodynamic instability due to long-term use of diuretic and antihypertensive drugs might stimulate ischemic change associated with MMVs. Our patient was negative in anticardiolipin antibodies and VDRL, and had no clinical features indicating antiphospholipid syndrome. She also had no evidence of thromboembolic disease. Her angiographic findings showed typical of MMD and we could speculate a related MMS following her SLE, even though no SLE disease activity was detected for 2 years. Also, we suggest a hidden genetic factor may play a role in her MMS, although she has no reported family history of MMD.
Of the six patients diagnosed with MMS associated with SLE including our case, all were Asian including 3 Koreans, one Japanese, one Saudi Arabian and one Chinese, and five were female2,5-8). The known strong prevalence of MMD in Asian, especially in Japan, suggests that a genetic trait associated with the disease14). MMD occurs also more frequently in female, with a female to male ratio of 1.8 to 2.18:1, and peak incidences are at aged 1-10 and 30-50. The bimodal age distributions as well as genetic trait of MMD may affect a diverse interval between initial SLE and following MMS.
In conclusion, we present an unusual case of MMS in a girl with SLE who was successfully treated through the bypass surgery. MMD is observed more frequently in Asia. Therefore, whenever an SLE patient presents symptoms of CVA either with or without disease activity, the related or accidental MMD should be included in the differential diagnosis, particularly in prevalent areas. Additionally, we would like to make an overture that an initial cerebrovascular check up might be helpful to patients of the SLE in order to discover an unusual association of the MMD with the SLE thereafter.
Acknowledgments
This study was supported by a 2012 research grant from Pusan National University Yangsan Hospital.
Notes
No potential conflict of interest relevant to this article was reported.