Urinary 6-sulfatoxymelatonin level in girls and its relationship with obesity
Article information
Abstract
Purpose
Short sleep duration is associated with obesity. Urinary 6-sulfatoxymelatonin (6-OHMS), the principal metabolite of melatonin, is closely related with sleep. We evaluated the difference in urinary 6-OHMS levels between obese girls and normal weight girls, and the relationship of urinary 6-OHMS with other hormones regulating body weight and metabolism.
Methods
A total of 79 girls (6.3 to 12.4 years) were included in this study, of whom 34 were obese; 15, overweight; and 30, normal-weight. We examined their pubertal status and bone age. Fasting serum levels of total ghrelin, leptin, insulin, and first morning urinary 6-OHMS were measured. Homeostatic model assessment-insulin resistance (HOMA-IR) was calculated from the fasting insulin and glucose levels.
Results
There was no significant difference in the creatinine adjusted 6-OHMS levels between the obese girls and the control group. Urinary 6-OHMS did not show any correlations with body mass index (BMI), BMI percentile, total ghrelin, leptin, and HOMA-IR. Negative correlations were found between urinary 6-OHMS levels and chronological and bone ages.
Conclusion
Our results suggest that melatonin production is not reduced consistently in obese girls.
Introduction
Obesity has become a serious worldwide health problem. Obese children and adolescents are at risk of adult obesity and early development of obesity-associated health problems such as insulin resistance, type 2 diabetes, dyslipidemia, cardiovascular derangement and non-alcoholic fatty liver disease. It is well known that an imbalance of energy intake and expenditure results in obesity. These days, children and adolescents as well as adults have easy access to high calorie foods and their physical activities are decreased. Recently, many epidemiologic studies showed that short sleep duration is related with obesity in adults, adolescents, and children1-4). But the mechanism on how short sleep duration cause obesity is not yet known.
Melatonin is a pineal hormone that regulates the sleep-wake cycle. Its serum concentration shows a diurnal pattern; low in daytime and high in nighttime. Most of the melatonin is metabolized to 6-sulfatoxymelatonin (6-OHMS) in the liver and excreted in the urine. Urinary 6-OHMS levels correlate well with serum melatonin levels5,6).
Short sleep duration is related with low melatonin production7). Thus, melatonin may be the link between sleep duration and obesity. Administration of melatonin reduces body weight gain in rats8,9). MT2 melatonin receptors are present in human adipocytes10) and adipocyte differentiation is inhibited by melatonin11). But, there are few studies looking at melatonin levels in obese children and adolescents.
In this study, we evaluated the difference in concentration of the major melatonin metabolite, 6-OHMS in urine, in obese and normal weight girls. We also investigated whether 6-OHMS level is related with serum levels of total ghrelin, leptin and insulin resistance.
Materials and methods
1. Subjects
Seventy-nine Korean girls (6.3 to 12.4 years) were included in this study. They had visited the pediatric outpatient clinic of SMG-SNU Boramae Medical Center, Seoul, Korea, for evaluation of obesity or routine health check-ups from March 2008 to February 2010.
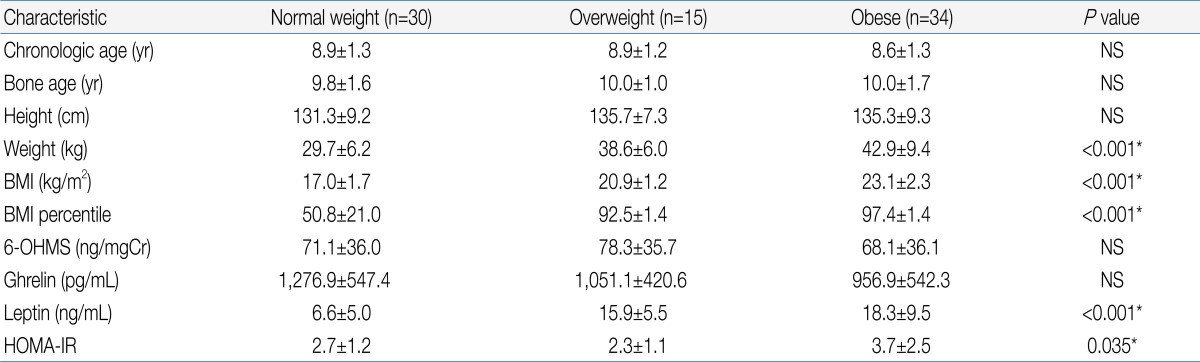
They were divided in three groups based on their body mass index (BMI, kg/m2); 1) obese group (BMI over 95th percentile, n=34): prepuberty (n=18), puberty (n=16); 2) overweight group (BMI over 85th percentile but less than 95th percentile, n=15): prepuberty (n=12), puberty (n=3); 3) normal weight group (BMI less than 85th percentile but over 15 percentile, n=30): prepuberty (n=17), puberty (n=13). There were no significant differences in the chronologic age, bone age and height between groups (Table 1). Girls who have pathologic obesity, diabetes, acute and chronic illness were excluded. Subjects whose Tanner stage is over 4 or who already had their menarche were also excluded. None of them had been taking any medication at the time of the study.
2. Methods
All of the subjects' height and weight were measured and the BMI was calculated as weight/height2 (kg/m2). Pubertal development was determined by physical examination using the Tanner stage. Bone age was evaluated by Greulich-Pyle method.
After a 12 hours overnight fast, blood samples and first morning urine samples were obtained. The urine samples were stored at -70℃ after centrifugation and the 6-OHMS and creatinine levels were measured at later date. Blood samples were centrifuged and the separated sera were stored at -70℃ and were later assayed for leptin and total ghrelin levels. Serum insulin and glucose levels were measured immediately at the hospital laboratory. HOMA-IR was calculated with them.
Urinary 6-OHMS concentrations were measured by enzyme-linked immunosorbent assay (Bühlmann Laboratories AG, Schönenbuch, Swiss) and urine creatinine levels were determined by colorimetry (Assay Designs Inc., Ann Arbor, MI, USA). Both serum levels of leptin and total ghrelin were measured by radioimmunoassay (Linco Research, St. Charles, MO, USA). Serum insulin assay was done by immunoradiometric assay (DIAsource, Nivelles, Belgium).
HOMA-IR was calculated as follows;
HOMA-IR=fasting insulin (µU/mL)×fasting glucose (mmol/L)/22.5
3. Statistics
We compared the urinary 6-OHMS, serum leptin, serum total ghrelin levels and HOMA-IR between the obese group and normal weight group. Results were analyzed by analysis of variance (ANOVA), Student t-test and Mann-Whitney U test. To find any relationship of urinary 6-OHMS levels with the demographic factors and hormonal levels, Pearson's correlation analyses were done using all the 79 girls including the overweight group. SPSS ver. 15 (SPSS Inc., Chicago, IL, USA) was used. All data were expressed as mean with standard deviation. P value<0.05 was considered statistically significant.
4. Ethics statement
This study was approved by the Institutional Review Board (IRB) of the SMG-SNU Boramae Medical Center. Informed consent was confirmed by the IRB.
Results
1. Urinary 6-OHMS concentration of obese and normal weight girls
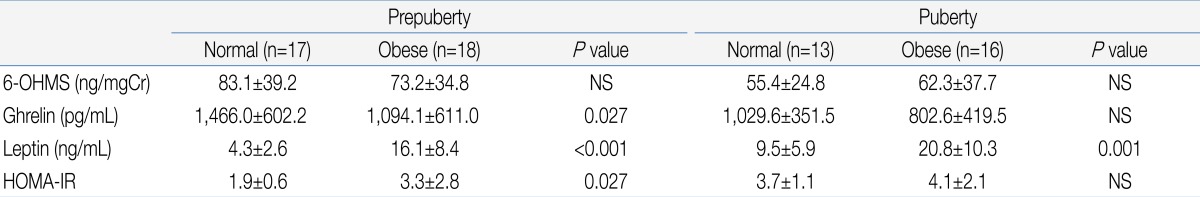
There was no significant difference in urinary 6-OHMS concentration between the obese group and normal weight group (Table 1). When the subjects were divided according to the presence or absence of onset of puberty, there was also no significant difference in urinary 6-OHMS concentration between the obese and normal weight girls in both the pre-pubertal and pubertal groups (Table 2).
2. Relationship of 6-OHMS with demographic parameters
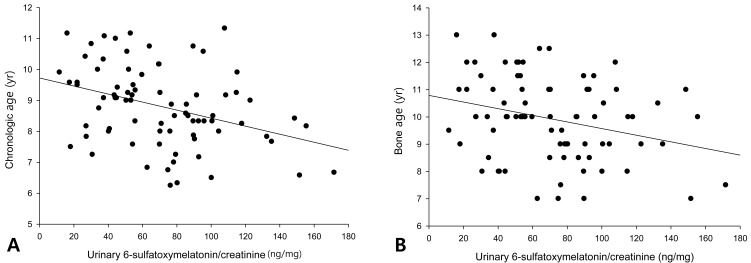
The urinary 6-OHMS levels adjusted by creatinine levels of the 79 girls showed no correlation with the BMI (Fig. 1A) or BMI percentile (Fig. 1B). The urinary 6-OHMS levels showed negative relationships with chronologic age (CA) (Fig. 2A) and bone age (BA) (Fig. 2B).
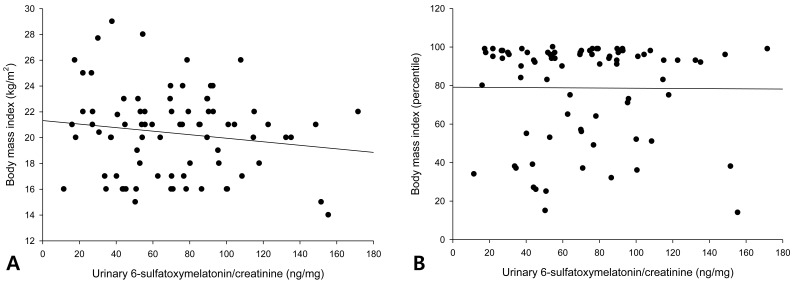
No correlations between urinary 6-sulfatoxymelatonin and either body mass index (BMI) (A) or BMI percentile (B).
3. Relationship of 6-OHMS with endocrine parameters
There was a positive correlation between urinary 6-OHMS levels and total ghrelin levels (r=0.25; P=0.03) of the total 79 girls. But after controlling for chronologic age or bone age, the correlation between urinary 6-OHMS levels and serum total ghrelin levels disappeared. No correlations were evident between urinary 6-OHMS levels and serum leptin levels and HOMA-IR.
Discussion
We evaluated whether melatonin levels were different in obese and normal weight girls by measuring 6-OHMS, a major metabolite of melatonin. First morning urine samples were collected for analysis. Creatinine adjusted 6-OHMS levels in the first morning urine correlated with the 6-OHMS levels in 24 hours or nighttime urine and represented serum concentrations of melatonin during the nighttime5,12).
6-OHMS levels showed large inter-individual variation as in a previous study13), but were not different between the obese and control group. BMI did not correlate with 6-OHMS levels in our study. The results were similar in both pre-pubertal and pubertal girls.
Our initial hypothesis was that obese girls would have lower 6-OHMS levels than normal weight girls. But we did not find any difference in 6-OHMS level between the two groups. Fideleff et al.14) also reported that there was no significant difference of urinary 6-OHMS levels between obese and normal weight girls.
Our results revealed that there was no relationship between melatonin levels and obesity. But we didn't measure the actual sleep duration. Therefore, we could not conclude that sleep duration was not different between obese group and control group. In addition, due to the relatively small size of the study population and the large inter-individual variation of 6-OHMS levels, large population studies should be done to validate this result.
Furthermore, melatonin level can be affected by season, especially sunlight exposure time, and the amount of consumption of certain vegetables. A previous study revealed that melatonin levels in human did not change according to the season13). Consumption of the melatonin containing vegetables such as sweet corn, bitter gourd, Japanese radish sprout, garland chrysanthemum, shimeji mushroom and shiitake mushroom may affect the 6-OHMS level15). Our study did not control for seasonality nor the kinds of food being ingested. It is possible that the variable levels of 6-OHMS obtained resulted from the variety of melatonin containing foods being consumed by each individual.
Melatonin level is known to decrease with increasing age16). The serum melatonin concentration at night was low during the first 6 months of life. It reached to peak level at 1 to 3 year of age, and decline thereafter. The reason for this was supposed to be the increased body size in children and adolescents and constant size of pineal gland after infancy17).
In this study, correlation analysis of 79 girls revealed that CA or BA were inversely related to 6-OHMS levels. It was not the primary aim of this study to compare leptin levels, total ghrelin levels, and HOMA-IR between obese girls and controls. By ANOVA, there were significant differences in leptin and HOMA-IR among obese, overweight, and normal weight groups. As we expected, the serum total ghrelin levels were lower and serum leptin levels were higher in the obese group than in controls. HOMA-IR seemed to be increased in the obese group but they were not significant in our subjects.
Melatonin was known to not only be a regulator of the circadian rhythms, but also have antioxidant properties and immune effects18). There are many animal studies to show the effect of melatonin on energy metabolism. It was reported that melatonin administration improved fatty liver19), and dyslipidemia20) in rats. Absence of melatonin increased insulin resistance21) and removal of melatonin receptor 1 induced insulin resistance22). Also, administration of melatonin improved glucose homeostasis in mice23) and reduced insulin level or glucose level8,9,24). On the contrary, melatonin levels in human are not related to insulin levels in normal subjects, although patients with metabolic syndrome show a significant correlation between melatonin and insulin levels25). Moreover, patients with metabolic syndrome who were treated with melatonin showed improvement of blood pressure, lipid profile and parameters of oxidative stress26).
However, our study showed no significant correlation between 6-OHMS levels and HOMA-IR. This result was probably because most subjects in our study did not have severe metabolic derangement. If we investigate the relation of 6-OHMS levels and HOMA-IR in patients with metabolic syndrome, the result may be different.
Leptin and ghrelin are two peripheral hormones regulating appetite and energy metabolism. They have functionally reciprocal properties each other. Leptin produced by adipose tissue, reduces food intake through delivery of information about peripheral energy status to the hypothalamus. Ghrelin which is produced and secreted mainly by the stomach increases appetite, and its serum level is closely related to food intake.
In obese people, the serum levels of leptin are higher and the serum levels of ghrelin are lower than in normal weight people. Melatonin administration reduced leptin levels in rat8,9). But the melatonin effect on ghrelin is controversial. It was reported that exogenous melatonin reduced serum level of ghrelin in rat27). Other animal studies showed that melatonin administration increased immunohistochemical staining of hypothalamic ghrelin, but did not alter the staining of stomach ghrelin and serum ghrelin levels28,29).
In this study, we could not find any relationship between 6-OHMS and leptin and ghrelin level. As mentioned above, this study has some limitations; First, this study was a cross-sectional study for relatively small number of girls with a limited age. If we studied for much more girls and including boys, we might see the different or more evident results. Second, we didn't evaluate the actual sleep duration. We investigated the level of urinary 6-OHMS as a representative of sleep duration or quality. But it is uncertain that only one morning urine sample was adequate to represent the usual daily sleep duration or serum melatonin level. Third, we didn't control melatonin consumption as food. The amount of melatonin containing food consumption might affect the individual results.
In conclusion, melatonin production is not reduced in obese girl compared with the normal weight girls. Further studies for the relationship with sleep, melatonin and energy metabolism are required.
Acknowledgment
This study was supported by a grant from SMG-SNU Boramae Medical Center.