Association of gut microbiota with obesity in children and adolescents
Article information
Abstract
Pediatric obesity is among the most serious global health problems whose prevalence has increased over the past decade. Pediatric obesity increases concomitant health problems, including type 2 diabetes mellitus, hypertension, dyslipidemia, fatty liver disease, and psychological problems, which often progress into adulthood. The gut microbiota is a new factor in the development of obesity, which is affected by renowned risk factors such as diet, lifestyle, and socioeconomic status. This review aimed to describe the association between the gut microbiota and childhood obesity. According to advances in gene sequencing technologies, many findings of experimental animal and human studies of adults and children demonstrated that compositional and functional changes in the gut microbiota (dysbiosis) are associated with the development of obesity. Many studies have reported that an increased Firmicutes/Bacteroidetes (F/B) ratio is a biomarker of obesity susceptibility; however, with the rapid accumulation of data, meta-analyses of human gut microbiota and obesity showed no clear association between F/B ratio and obesity status. The contribution of the microbiota to obesity has been considered using multifactorial approaches, such as supplying additional calories to the host, modulating blood lipopolysaccharide levels, favoring fat storage, and affecting satiety. Probiotics are proposed to manipulate the gut microbiota population to improve obesity; however, their clinical application remains limited because trials have shown different results. Further studies are required to better understand the mechanisms underlying the observed association between the gut microbiota and pediatric obesity.
Key message
The gut microbiota is an emerging factor in the development of pediatric obesity, which is affected by renowned risk factors such as diet, lifestyle, and socioeconomic status. This review aimed to describe the association between the gut microbiota and childhood obesity.
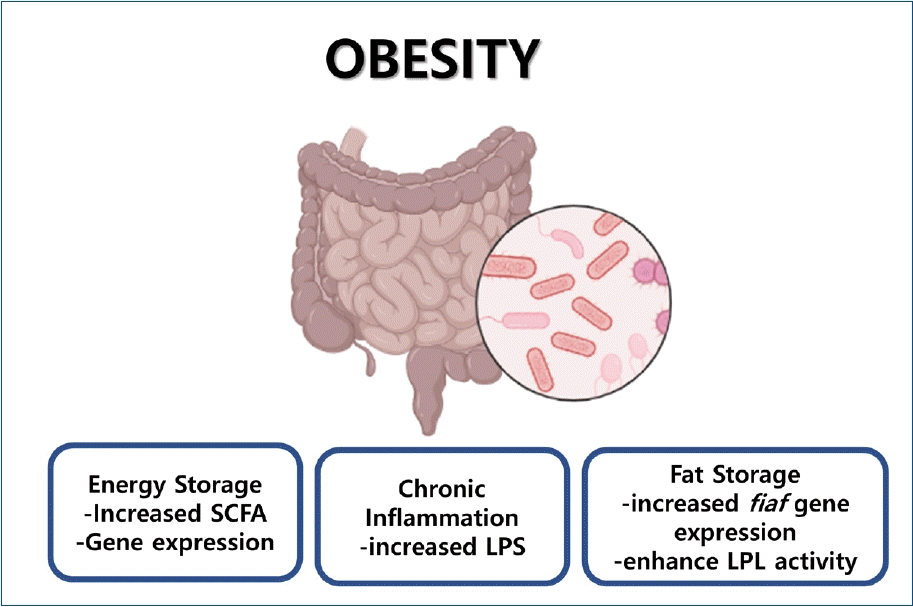
Graphical abstract. The proposed mechanism of obesity induced by the gut microbiota has been considered using multifactorial approaches, such as supplying additional calories to the host by short-chain fatty acids (SCFA), increasing blood lipopolysaccharide (LPS) levels leading to chronic inflammation, favoring fat storage by increasing fiaf gene expression, and enhancing lipoprotein lipase (LPL) activity.
Introduction
Pediatric obesity is among the most serious global health problems whose prevalence has increased over the past decade [1]. As a consequence of school closures during the coronavirus disease 2019 pandemic, the prevalence of pediatric obesity after the pandemic has increased abruptly [2]. Pediatric obesity increases one’s concomitant health problems, including type 2 diabetes mellitus, hypertension, dyslipidemia, fatty liver disease, and psychological problems, that often persist into adulthood [3]. Disruption of the normal equilibrium between the gut microbiota and the host, known as dysbiosis, may affect metabolic pathways, resulting in the development of obesity and other metabolic disorders [4]. The abundance of fiber-metabolizing microbes is associated with weight loss, while that of protein and fat-metabolizing bacteria is associated with weight gain [5]. Insulin resistance is correlated with lower alpha and beta diversity in children, showing less species diversity in obese children with higher Homeostatic Model Assessment for Insulin Resistance levels [6]. This lower alpha and beta diversity were also found in children with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis compared to normal-weight children [7]. Obesity is a major risk factor for metabolic diseases.
Several studies have shown that obesity is associated with gut microbial alterations [8]. Advances in the next-generation sequencing techniques of the 16SrRNA gene have increased our ability to study the development of gut microbiota and its association with health outcomes [9]. This review aimed to describe the association between pediatric obesity and gut microbiota. Additionally, the proposed mechanisms of obesity induced by gut microbiota and microbiota manipulation in the treatment of obesity will be discussed.
Association between obesity and gut microbiota
Genetic disposition, diet, and a sedentary lifestyle are the major factors that determine childhood obesity. According to recent advances in gene sequencing technologies, extensive findings of experimental animal and human studies have demonstrated that compositional and functional changes in the gut microbiota, termed as dysbiosis, are associated with the development of obesity [8]. Alterations in diversity and microbiota community structure may affect a host’s metabolism, resulting in obesity [10].
The first evidence of an obesogenic gut microbiota profile was obtained from animal studies. Ley et al. [11] discovered differential gut microbial compositions in genetically obese (ob/ob) mice versus their lean (ob/+) and wild-type (+/+) siblings under the same polysaccharide-rich diet, with findings of reduced relative abundance of Bacteroidetes and increased Firmicutes in obesity. Moreover, Turnbaugh et al. [12] transplanted lean and obese microbiota into germ-free mice and found a significantly greater increase in total body fat in mice colonized by obese microbiota than lean microbiota. Animal models have provided evidence of an association between gut microbiota and obesity.
Consistent with animal models, a human cross-sectional study revealed higher proportions of Firmicutes and relatively fewer Bacteroidetes in 12 obese volunteers versus lean adult controls [13]. Another study also showed a decrease in Bacteroidetes in 9 patients with anorexia nervosa and 20 normal-weight healthy controls versus 20 obese subjects [14]. Moreover, interventional studies of weight reduction programs demonstrated a relationship between gut microbiota and obesity [15]. Some reports have demonstrated that weight reduction by bariatric surgery partially reversed obesity-associated microbial alterations [16,17].
Regarding pediatric obesity, most studies of the gut microbiota were cross-sectional in design. Previous studies of Hispanic and Mexican children revealed significant differences in microbiota composition between obese and lean children [18,19]. A cross-sectional study of Korean children also showed an association between the gut microbiota and pediatric obesity [20]. Recent studies demonstrated that factors affecting young infant overgrowth may be mediated by gut microbiota profiles [21]. One prospective study of children that investigated microbial changes associated with weight gain over a 4-year period showed that the microbiota–host–diet configuration could be a predictor of obesity [22]. Another study of Korean children revealed lifestyle modifications with a 2-month weight reduction program that resulted in alterations in the composition and function of the gut microbiota in the obese group [23].
Although many studies have investigated the association between obesity and gut microbiota, it is difficult to conclude that the observed dysbiotic microbiota is the cause rather than the consequence of such diseases [24].
Gut microbiota profile in obesity
1. Firmicutes to Bacteroidetes ratio
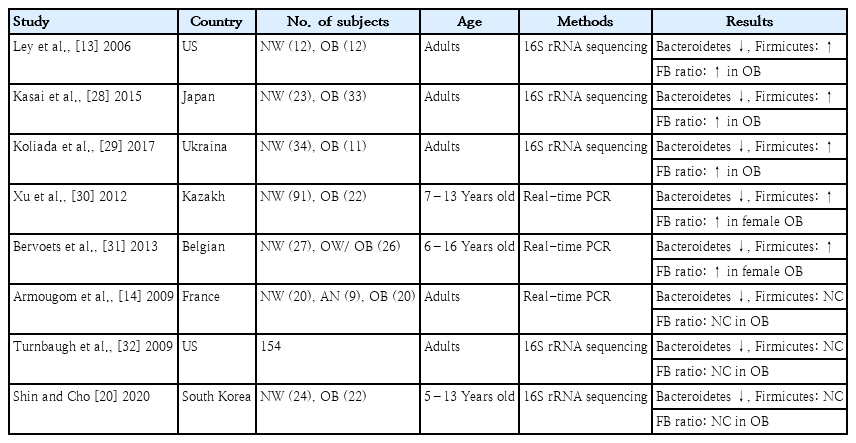
The 2 most important phyla in the gastrointestinal tract are Firmicutes and Bacteroidetes, which represent 90% of the gut microbiota [13]. Firmicutes bacteria are gram-positive and play a key role in host nutrition and metabolism through the synthesis of short-chain fatty acids (SCFA). Through their metabolic products, Firmicutes bacteria are indirectly connected with other tissues and organs and regulate hunger and satiety [25]. In contrast, Bacteroidetes bacteria are gram-negative and associated with immunomodulation [26]. The Firmicutes to Bacteroidetes (F/B) ratio is widely accepted as playing an important role in maintaining intestinal homeostasis [25]. An association between an increased F/B ratio and obesity has been reported in humans. Ley et al. [13] first reported that the relative proportions of Bacteroidetes are decreased in obese versus lean people. A recently reported systematic review revealed that most studies supported this relationship [27]. A study of adults revealed significant differences in F/B ratios in fecal samples between obese and nonobese Japanese subjects [28]. Similar findings among Ukrainian adults reported that F/B ratio was significantly associated with body mass index (BMI) [29]. In a study of Kazakh and Belgian school children, the F/B ratio was significantly higher in the obese versus control groups [30,31].
In contrast, the F/B ratio was seemingly a biomarker of obesity susceptibility at the start of a study on the gut microbiota and obesity, but several studies revealed no relationship between them (Table 1). A diminished proportion of Bacteroidetes was also observed in obese versus normal-BMI and anorexic patients, whereas Firmicutes exhibited no differential abundance [14]. A further extensive metagenomics study resolving the gut microbiota in obese and lean twins revealed lower bacterial diversity in the Bacteroidetes proportion in obese individuals compared toean individuals, whereas the proportional difference in Firmicutes was insignificant [32]. A cross-sectional study of Korean children suggested that the relative abundance of Bacteroidetes, but not Firmicutes, was decreased in the obese versus normal-weight group [20]. Conflicting findings could be explained by the heterogeneity in age, genetic background, ethnicity, lifestyle, and diet of subjects across studies. Another conflicting factor to consider is the type of metabolic components affected, such as insulin intolerance, hyperlipidemia, and hypertension, in the study group. However, with the rapid accumulation of data, meta-analyses of human gut microbiota and obesity could not identify a clear trend between F/B ratio and obesity status [26].
2. Specific bacteria related to obesity
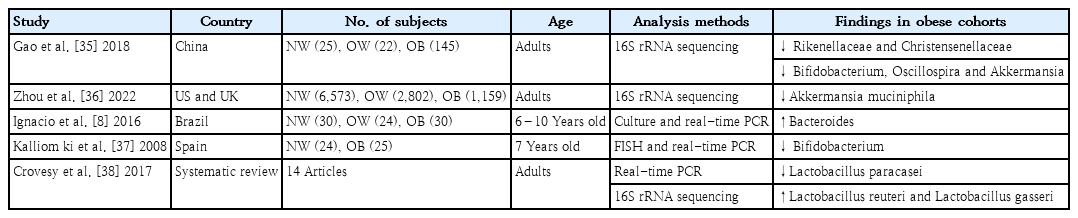
Studies to date have indicated that obesity may be associated with shifts in intestinal bacteria at the phylum level. However, discrepancies exist in the directionality and relevance of the F/B ratio in obesity [33]. Reported shifts at the phylum level do not completely capture the compositional changes in the gut microbiota associated with obesity [34]. Therefore, many studies have attempted to identify the key bacteria at lower taxonomic levels (family, genus, and species) rather than the phyla Firmicutes and Bacteroidetes, which play a key role in the development of obesity (Table 2).
The most common gut microbiota composition finding was a reduction in butyrate-producing microbes and an increase in opportunistic pathogens. Consistent microbiota findings have displayed reductions in the abundances of the families Rikenellaceae and Christensenellaceae, as well as a decrease in the abundance of the genera Bifidobacterium, Oscillospira, and Akkermansia [35]. Akkermansia muciniphila is a known beneficial bacteria in obesity and associated metabolic disorders [36]. Bacteroides is reportedly prominent among obese individuals, and its abundance is positively correlated with BMI [8]. A lower fecal Bifidobacterium load during early infancy is found in obese children compared to normal-weight control [37]. The abundance of Lactobacillus paracasei was negatively correlated with obesity, while the abundances of Lactobacillus reuteri and Lactobacillus gasseri were significantly correlated with obesity [38]. That study revealed that obesity-related microorganisms are species-specific in the same genus. However, no single genera or species play a proven key role in obesity [39]. One major concern is that a proven causative relationship between key bacteria and disease is largely lacking.
Proposed mechanism of obesity induced by gut microbiota
Different mechanisms have been proposed to explain the relationship between the gut microbiota composition and the development of obesity [40]. The first mechanism relates to the role of gut microbiota in extracting energy from nondigestible carbohydrates through metabolic products [41]. The most abundant metabolic products of the gut microbiota are SCFA, mainly acetate, propionate, and butyrate, which are produced by anaerobic fermentation of nondigested carbohydrates [42]. Cecal gut microbiota from obese versus lean mice are enriched in enzymes that break down nondigestible carbohydrates by the host, leading to increased production of SCFA involved in energy storage [4,12]. In rodents, postbiotics, mainly SCFA, may increase energy expenditure by inducing thermogenesis in brown adipose tissue and browning white adipose tissue [43]. A human pilot study revealed that butyrate oral supplementation beneficially affects glucose metabolism in lean but not metabolic syndrome individuals, presumably due to altered SCFA handling in insulin-resistant subjects [44]. SCFA play a crucial role in the development of obesity by providing an extra source of calories to the host, but conflicting results have been found that warrant further research.
The second mechanism refers to the ability of the gut microbiota to modulate blood lipopolysaccharide (LPS) levels by disrupting epithelial barrier integrity, triggering the onset of moderate systemic chronic inflammation that predisposes the host to obesity [45]. In mice, a high-fat diet increased the proportion of an LPS-containing gut microbiota, leading to a 2–3× increase in plasma LPS concentrations [46]. That study also reported that a 4-week continuous subcutaneous LPS infusion perturbed glucose tolerance by inducing hepatic insulin resistance and hampering glucose-stimulated insulin secretion [46]. Associations between LPS concentrations and several aspects of metabolic syndrome were also noted in a systematic review of human studies [47].
The third mechanism relies on the fact that the gut microbiota can regulate host genes associated with energy storage and consumption that favor fat storage [48]. The gut microbiota can also affect appetite and satiety via vagal nerve activation or immune-neuroendocrine mechanisms [49]. It also promotes bile acid metabolism and modifies hepatic triglyceride and glucose homeostasis through the farnesoid X receptor [50]. The gut microbiota inhibits fiaf gene expression, enhancing lipoprotein lipase activity and ultimately resulting in increased lipid storage within white adipose tissue [51]. Modulation of the gut microbiota with different diets and supplementation with probiotics and dietary fibers is a promising approach to treating and preventing obesity.
Microbiota manipulation in treatment of obesity
1. Probiotics for obesity
Probiotics are also proposed methods to manipulate the gut microbiota population and improve metabolic conditions. According to the World Health Organization, probiotics are live microorganisms that can confer health benefits to the host when administered in adequate amounts [52]. Probiotics administration is a strategy for altering gut microbiota composition and preventing and treating several pediatric diseases, including obesity, inflammatory disease, and atopic diseases. However, its effectiveness and mechanisms of action have not been fully elucidated. Previous clinical trials aimed to determine the ability of prebiotics, probiotics, and synbiotics to reduce weight. A randomized controlled trial of adults showed that the modification of gut microbiota composition by probiotics could reduce body weight [53]. Supplementation with A. muciniphila improved metabolic parameters in overweight and obese subjects [54]. However, investigations of microbiota changes in the association between probiotic and prebiotic administration and pediatric obesity are rare. Lactobacillus paracasei F19 administration to 120 infants during weaning from 4 to 13 months of age did not modulate their body composition or growth at school age [55]. However, the administration of probiotics/prebiotics mixture to overweight and obese children significantly decreased their body fat and significantly increased their fecal Bifidobacterium spp. loads [56]. Two randomized clinical trials showed that administration of synbiotics for 4 and 8 weeks to primary obese children significantly reduced their body weight and BMI z score and changed the results of other anthropometric analyses [57,58]. The different results may be associated with various strains, races, ages, and intervention durations. Probiotic/synbiotic administration alters gut microbiota and may contribute to controlling pediatric obesity; however, further research is required to determine its clinical effects. Also, the clinical application of probiotics in obesity remains limited because these trials showed different results.
2. Fecal microbiota transplantation for obesity
Based on the fact that dysbiosis of gut microbiota results in pediatric diseases, strategies to restore gut microbiota dysbiosis have been widely investigated. Fecal microbiota transplantation (FMT) is a strategy to manipulate the entire gut microbiota based on the idea that the microbiota is a cause of obesity. FMT procedures involve the transfer of screened fecal material containing microbiota from healthy donors to obese patients. An FMT from a single lean vegan-donor FMT to obese male patients with metabolic syndrome changed their intestinal microbiota composition without significantly changing their BMI [59]. A recent study using FMT via oral capsules from a single lean donor showed that obese patients sustained shifts in microbiota associated with obesity toward those of the lean donor without significant changes in mean BMI [60]. These trials demonstrate the beginning steps of FMT for weight loss in obese patients. However, because the causal relationships between the gut microbiota and obesity are unclear, the effect and mechanism by which FMT influences obesity remain unknown [61].
Other factors related to gut microbiota contributing to childhood obesity
Different factors related to gut microbiota abundance, such as gestational weight gain, perinatal diet, antibiotic administration to the mother and/or child, type of delivery, and feeding patterns, seem to contribute to childhood obesity [62,63]. Maternal gestational weight gain influences gut microbiota acquisition, composition, and microbial activity during early infancy [64]. Maternal diet during gestation and lactation modulates the abundance and prevalence of the infant’s microbiota, thus changing the bacterial pool transferred to the offspring during pregnancy and early life [65]. The current study revealed that antibiotic use-related disturbances in gut microbiota richness and diversity may be a possible mediating mechanism in obesity [66]. Genus- and species-level gut microbiota differed among children born by cesarean section versus vaginal delivery [67]. The microbial features of formula-fed infants increases their risk of being overweight compared to breastfed infants [68]. Childhood obesity is a multifactorial complex disease associated with the gut microbiota.
Conclusion
The gut microbiota is a new factor in the development of obesity that is affected by renowned risk factors, such as diet, lifestyle, and socioeconomic status. Although the complicated relationships between the gut microbiota and obesity using compositional data remain inclusive, modulating gut microbiota through diet and food supplements to alleviate obesity-related symptoms is challenging. Further studies are required to better understand the mechanisms underlying the observed association between the gut microbiota and obesity.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This review was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2021R1F1A1058240).