Management of patients with allergic diseases in the era of COVID-19
Article information
Abstract
In the early days of the coronavirus disease 2019 (COVID-19) pandemic, allergic diseases, especially asthma, were considered to be risk factors for severe COVID-19 infection, hospitalization, and death. These concerns stemmed from the idea that individuals with allergic diseases are generally more susceptible to respiratory virus infections, which are major causes of exacerbation of allergic diseases. However, epidemiologic data with mechanistic studies showed that the associations between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and clinical outcomes of allergic diseases are complex and affected by diverse factors such as allergic disease severity, phenotypes, and control status with current medications. In addition, children generally have less severe clinical outcomes of COVID-19 than those of adults, which complicates the association between allergic diseases and COVID-19-related outcomes among them. The present review summarizes the potential association between allergic diseases and COVID-19-related outcomes and discusses the factors requiring consideration. The findings viewed herein will aid the management of allergic diseases in patients with SARS-CoV-2 infection and the establishment of medical polices for managing patients with allergic diseases.
Introduction
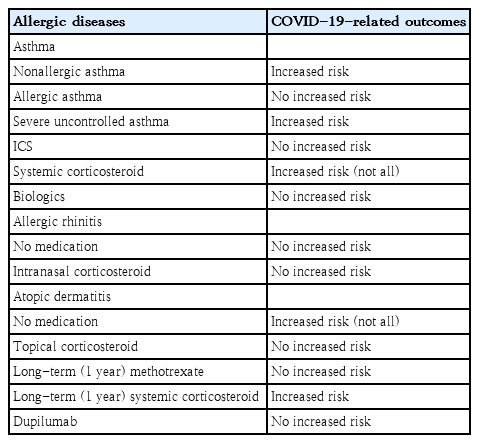
Individuals with allergic diseases, particularly asthma, are more susceptible to respiratory virus infections, which are the leading causes of exacerbations of allergic diseases [1]. Accordingly, in the early days of the coronavirus disease 2019 (COVID-19) pandemic, patients with allergic diseases were considered to have higher risk for COVID-19-related morbidity and mortality [2]. However, accumulating evidence suggests that allergic diseases are not always associated with poor clinical outcomes of COVID-19 and that the relation between allergic diseases and COVID-19 is rather complex. Considering the relatively high prevalence of allergic diseases, a considerable number of patients with them become infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Various factors of allergic diseases, including phenotypes, medications, and control status, should be considered when predicting the clinical outcomes of COVID-19 in patients with allergic diseases. The present review summarizes the complex relationship between COVID-19-related outcomes and allergic diseases (Table 1) to aid the management of SARS-CoV-2 infections among patients with allergic diseases.
Asthma
Since the beginning of the COVID-19 pandemic, patients with asthma and their caregivers have been concerned about increased susceptibility to SARS-CoV-2 infection and severe COVID-19. The COVID-19 pandemic has affected the patterns of visits to medical institutions during asthma exacerbations. Patients with more severe asthma visited emergency departments and were hospitalized more often during versus before the pandemic [3]. To better predict the clinical outcomes of patients with asthma who become infected with SARS-CoV-2, several factors including the medical history, severity, and treatment of individual asthma cases should be considered. Moreover, to manage asthma during the COVID-19 pandemic, each patient’s asthma phenotype and endotype require elucidation.
1. Association between asthma and clinical outcomes of COVID-19
Viral respiratory infections are common causes of asthma exacerbation in children and adults. Several studies have reported a substantial reduction in asthma exacerbation during the COVID-19 pandemic [4-10]. However, whether SARS-CoV-2 infections cause or are associated with acute exacerbation in patients with asthma remains controversial [11]. Such associations might be independent of improved asthma control during the COVID-19 pandemic considering possible confounding factors such as social distancing, minimal social interactions, and lower susceptibility of younger individuals to SARS-CoV-2 infection [5,12]. To date, few studies have reported that SARS-CoV-2 infection can trigger asthma exacerbation but does not cause severe asthma exacerbation [13-15]. Further large-scale studies are needed to confirm these associations between SARS-CoV-2 infection and asthma exacerbation.
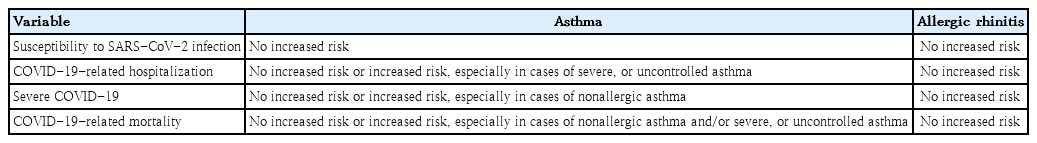
In the early stages of the COVID-19 pandemic, the effect of asthma on COVID-19 severity in children was examined in small studies, partially because of the lower prevalence of COVID-19 in children [16]. Whether asthma is a risk factor for COVID-19-associated hospitalization or severe COVID-19 remains controversial. Some studies have reported that asthma is not a risk factor for severe COVID-19 (Table 2) [16,17]. However, others reported that children (12–17 years) with asthma are at higher risk of requiring hospitalization for COVID-19 [18]. It has also reported that COVID-19 patients aged ≥16 years with asthma are more likely to require critical care (adjusted odds ratio [aOR], 1.20; 95% confidence interval [CI], 1.05–1.37), noninvasive ventilation (aOR, 1.36; 95% CI, 1.18–1.57), and oxygen supplementation (aOR, 1.33; 95% CI, 1.17–1.50) than those without asthma [19]. These findings suggest that the association between asthma and severe COVID-19 is complex and that the characteristics of the study population require consideration to reveal the potential connections.
2. Effect of asthma severity and phenotypes on clinical outcomes of COVID-19
Asthma severity and control status affect COVID-19 severity. Because the prevalence of severe asthma in children is relatively low compared with that in adults [20], few studies have reported the effects of severe asthma on the clinical outcomes of COVID-19 in adolescents. Children aged 12-17 years with asthma prescribed one (adjusted hazard ratio [aHR], 2.58; 95% CI, 1.82–3.66) or 2 or more (aHR, 3.80; 95% CI, 2.41–5.95) courses of oral corticosteroids in the year preceding the COVID-19 pandemic were reportedly at greater risk of COVID-19-related hospitalization [18]. In adults, uncontrolled asthma (OR, 1.64; 95% CI, 1.35–2.00) and the requirement of higher amounts of asthma medications, such as high-dose inhaled corticosteroid (ICS) combined with long-acting β-agonists (OR, 1.40; 95% CI, 1.22–1.60), was associated with severe COVID-19.21) Also, in patients aged ≥16 years, severe asthma significantly increases COVID-19-related mortality rates (aHR, 1.96; 95% CI, 1.25–3.08) [19].
One reason for the ongoing debate about the association between asthma and COVID-19 outcome is the difference in asthma phenotype and endotype among patients [17,22]. Allergic asthma, compared with nonallergic asthma, is associated with a lower risk of COVID-19-related hospitalization [22]. Moreover, nonallergic asthma is associated with severe COVID-19, while allergic asthma is not [22]. Although the mechanisms underlying these associations are unclear, the relation between angiotensin-converting enzyme 2 (ACE2) receptor expression in the airway and the degree of Th2 inflammation with genetic predisposition may provide some clues [22-24].
3. Effect of asthma medications on clinical outcomes of COVID-19
ICS is the mainstay of asthma medications [25]. However, concern exists regarding whether ICS use is associated with an increased risk of susceptibility to SARS-CoV-2 infection or severe COVID-19 due to the potential immunosuppressive effects of ICS through increased susceptibility to secondary infection, viral replication promotion, and delayed viral clearance [26,27]. However, ICS can be safely used in patients with asthma even during the COVID-19 pandemic and SARS-CoV-2 infection [28]. Several studies have reported that patients aged 16–49 years using ICS with or without long-acting β-agonists do not carry an increased risk for COVID-19-related in-hospital mortality [19]. The proportion of ICS usage in adult asthma patients is significantly lower among those who are hospitalized due to COVID-19 than among those who are not [29]. Moreover, ICS use in adult asthma patients is not associated with COVID-19 related mortality [28]. The potential protective role of ICS against COVID-19 may be due to the suppressive effect of ICS on coronavirus replication [30], which can also support its use to treat COVID-19 itself. Furthermore, ACE2 and transmembrane serine protease 2 (TMPRSS2) gene expression levels are lower in asthma patients receiving ICS treatment [31]. Previous studies reported that ICS use is safe regardless of SARS-CoV-2 infection; therefore, ICS should be used as prescribed. The discontinuation of asthma medication is linked with poorly controlled asthma and decreases quality of life [32,33]. Therefore, maintaining a stable asthmatic status helps improve the clinical course and outcomes of COVID-19.
Studies on the safety of chronic or regular systemic corticosteroid treatment in COVID-19 patients remain scarce, and the results of the few studies to date are inconclusive. A need for systemic corticosteroid treatment indicates severe uncontrolled asthma associated with increased risks of severe COVID-19 and COVID-19-related mortality rates [2,34].
Allergic rhinitis
Since COVID-19 symptoms can often accompany upper respiratory tract infections, it can be difficult to distinguish from common cold and allergic rhinitis. The European Academy of Allergy and Clinical Immunology (EAACI) provides a set of criteria to distinguish COVID-19 from other rhinologic diseases using a modified Delphi process [35]. Smell dysfunction, taste dysfunction, dyspnea, and cough are predominant symptoms of COVID-19, whereas sneezing, stuffy nose, and nasal pruritus with ocular itch or redness predominantly indicate allergic rhinitis. For the common cold, a runny and stuffy nose are the most common symptoms. When more than 2 rhinologic diseases develop simultaneously, more diverse symptoms may present.
During the COVID-19 pandemic, medications used for allergic rhinitis treatment, including intranasal corticosteroids, have been safely administered to patients with allergic rhinitis and SARS-CoV-2 infection. Although no studies have examined the association between intranasal corticosteroid use and COVID-19-related disease burden in children, studies in adults showed that intranasal corticosteroid therapy is associated with a lower risk of severe COVID-19 outcomes, including hospitalization, intensive care unit admission, and mortality [36]. Similar patterns were observed when intranasal corticosteroids were administered to patients who had never been diagnosed with allergic rhinitis [36]. Therefore, intranasal corticosteroids can be safely used in the management of allergic rhinitis even during SARS-CoV-2 infection.
Atopic dermatitis
Atopic dermatitis is one of the most common chronic inflammatory skin diseases [37]. The association between atopic dermatitis and COVID-19 outcomes has been less commonly investigated [38]. During the COVID-19 pandemic, an increased incidence of skin irritation and exacerbation of atopic dermatitis was reported, particularly among healthcare workers with prolonged use of personal protective equipment and increased work-related stress levels. Some studies in adults showed that atopic dermatitis was associated with an increased risk of COVID-19 [39,40]. Conversely, others reported that patients with atopic dermatitis do not have a significantly increased risk of SARS-CoV-2 infection [41]. On the other hand, the risk of COVID-19-related hospitalization was significantly lower in children with atopic dermatitis, compared with those without atopic dermatitis, with no differences noted in the prevalence of COVID-19-related severity and critical care admission [42]. These findings underline the complex relation among SARS-CoV-2 infection, COVID-19 outcomes, and atopic dermatitis.
Furthermore, concern exists regarding the effects of medications used to treat atopic dermatitis on SARS-CoV-2 infection and COVID-19 outcomes. Treatment with methotrexate for 1 year was not associated with severe COVID-19, COVID-19-related hospitalization, or critical care admission among children [42]. However, use of systemic corticosteroids for 1 year was associated with an increased risk of COVID-19-related hospitalization [42]. Even during the COVID-19 pandemic, well-controlled status of atopic dermatitis with appropriate medication is important for its proper management.
Food allergy
Only few studies have investigated the association between food allergy and COVID-19. A recent report stated that food allergy is associated with a decreased risk of SARS-CoV-2 infection [43]. One plausible explanation might be behavioral changes in patients with food allergy, such as a decreased requency of eating out. Apart from the association between food allergy and COVID-19, emphasis was placed on appropriate strategies for the management of food allergy with anaphylaxis during the pandemic due to the restriction of access to medical facilities [44,45]. These strategies included the use of telemedicine to improve treatment decision-making, full-time carrying of epinephrine autoinjector devices, and avoidance of known allergens [44,45].
Possible mechanisms for protective effects of allergic diseases in COVID-19 era
Type 2 inflammation might play a role in the association among allergic diseases, protective role of the therapies for asthma and allergic rhinitis, allergen sensitization, individuals’ allergic phenotypes and endotypes, and COVID-19-related outcomes [46]. However, further research is needed to elucidate these associations. Expression levels of ACE2, the target of SARS-CoV-2 spike protein binding, are inversely correlated with type 2 cytokine levels, whereas TMPRSS2 gene expression levels are positively correlated with type 2 cytokine levels in the primary airway epithelial cells obtained from patients with or without type 2 asthma [23]. Moreover, an inverse correlation between allergic sensitization and ACE2 expression levels in the nasal epithelium was noted regardless of the presence of asthma [24]. In children with asthma, the degree of allergic sensitization is inversely correlated with ACE2 expression levels [24]. However, ACE2 expression levels are not reduced in patients with non-atopic asthma [24], suggesting that the protective effect of allergic diseases is limited to patients with type 2 inflammation.
Blood eosinophils, a type 2 inflammatory response marker, promote protective responses against respiratory viruses [47]. Patients with eosinophil counts >200/µL show a significantly decreased risk of COVID-19-related mortality regardless of the presence of asthma in adults [48]. Adults with asthma with absolute eosinophil counts >150/µL reportedly show decreased COVID-19-related mortality rates, which supports the observation of decreased COVID-19-related mortality in patients with type 2 asthma phenotype [49].
Differences in clinical features of COVID-19 between children and adults with potential mechanisms
SARS-CoV-2 infection in children has a milder clinical course and less severe morbidity and mortality than that in adults (Fig. 1) [50]. Although the reasons for age-related differences in susceptibility to and disease severity of COVID-19 remain poorly understood, several studies focused on the key host proteins involved in SARS-CoV-2 cellular entry, including ACE2, TMPRSS2, and furin protein [51], which may also play a role in the association between allergic diseases and SARS-CoV-2 infection. SARS-CoV-2 binds to ACE2 on human airway cells, and the subsequent activation of serine protease, such as TMPRSS2, stimulates the spike protein to allow cellular entry [52]. ACE2 gene expression in the nasal epithelium increases with age [53,54], which can explain the decreased susceptibility to and milder symptoms of COVID-19 in children compared with that in adults. Furthermore, TMPRSS2 expression levels are increased by circulating androgens, which suggests that men are significantly more likely to develop severe COVID-19 than women and pubertal children show more severe COVID-19 symptoms than prepubertal children [55]. Although additional research is needed, differences in the expression levels of SARS-CoV-2 binding factors according to age might partially explain the differences in susceptibility to SARS-CoV-2 infection and COVID-19 severity [51].
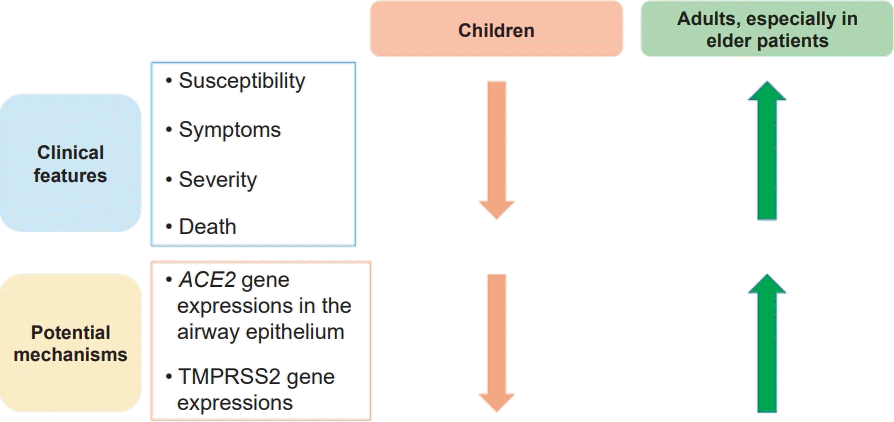
Comparison of clinical features of COVID-19 between children and adults with potential mechanisms. Children generally have less severe clinical outcomes of COVID-19 than those of adults. Differences in the expression levels of SARS-CoV-2 binding factors, such as ACE2, TMPRSS2, and furin protein, according to age might partially explain the differences in susceptibility to SARS-CoV-2 infection and COVID-19 severity. COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane serine protease 2.
Allergen immunotherapy in the era of COVID-19
Allergen immunotherapy can alter the clinical course of asthma and allergic rhinitis, even in children. Concern exists about the continuity of allergen immunotherapy in respiratory tract infections, including SARS-CoV-2 infection. A study on the effect of allergen immunotherapy in children infected with influenza virus indicated that children on maintenance doses of allergen immunotherapy show fewer symptoms and quick recovery from respiratory tract infections [56]. These findings suggest that allergen immunotherapy can be continued even during respiratory virus seasons, and likewise, during the COVID-19 pandemic [57].
The EAACI recommends administration of the COVID-19 vaccine at intervals of 7 days from subcutaneous allergen immunotherapy due to potential side effects [57]. In addition, it is recommended to discontinue sublingual immunotherapy 3 days before COVID-19 vaccination and resume 7 days after COVID-19 vaccination [57].
Biologics in the era of COVID-19
Biologics can be prescribed for uncontrolled severe asthma and moderate to severe atopic dermatitis, even in children and adolescents [58]. The biologics used in allergic diseases block type 2 inflammation pathways [58]. Some epidemiologic studies reported that biologics are not associated with an increased risk of COVID-19 [59] and that patients treated with biologics have a relatively low prevalence of COVID-19-related hospitalization [29]. Biologics used to treat asthma are not associated with increased risks of SARS-CoV-2 infection or severe COVID-19 [60-62]. In addition, dupilumab therapy for severe atopic dermatitis is safely and continuously used for better disease outcome, regardless of the presence of COVID-19 [63,64], as dupilumab does not affect antibody levels after mRNA vaccination for COVID-19 [65]. Based on the above mentioned findings, biologics can be safely used even during the COVID-19 pandemic.
COVID-19 vaccination in patients with allergic diseases
The age at which children can be vaccinated against COVID-19 has been gradually expanded, although more data are required to confirm the adverse reactions of COVID-19 vaccine in younger children [66]. COVID-19 vaccination can be safely administered in children and adolescents with allergic diseases except in cases of polysorbate or poly-ethylene glycol allergy and COVID-19 vaccine allergy. The pre-vaccine risk stratification algorithm can be used with a thorough history taking of adverse reactions to vaccines or other medications [67].
Evidence is limited on the potential effects of allergen immunotherapies and biologics targeting Th2 inflammation on anti-infectious vaccines [57]. A previous study showed that allergen immunotherapy has no effect on the booster vaccine dose for tickborne encephalitis [68]. Omalizumab therapy is well tolerated in response to live attenuated influenza vaccine [69]. Although further evidence is needed, allergen immunotherapy and biologics for allergic diseases can be safely used regardless of COVID-19 vaccination.
Conclusion
The risk of SARS-CoV-2 infection and severe COVID-19 outcomes are not elevated in patients with well-controlled asthma, particularly the type 2 phenotype of asthma and other allergic diseases. The mainstay treatment, i.e., ICS, intranasal corticosteroid, and topical corticosteroid can be safely used even in SARS-CoV-2-infected patients with allergic diseases. COVID-19 vaccine can also be safely administered to patients with allergic diseases. Moreover, allergen immunotherapy and biologics used to treat allergic diseases can be safely continued without concern about antibody responses.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was financially supported by Chonnam National University (Grant number: 2020–3735).