Should we prescribe carbapenem for treating febrile urinary tract infection caused by extended-spectrum β-lactamase-producing Enterobacteriaceae in children with vesicoureteral reflux?
Article information
Key message
Recent studies are focused on the noninferiority of noncarbapenem therapy for the treatment of extended-spectrum β-lactamases producing Enterobacteriaceae infections to reduce the utilization of carbapenem.
Urinary tract infections (UTIs) are the most common serious bacterial infections in children [1]. The most common pathogens causing UTIs are Enterobacteriaceae, such as Escherichia coli and Klebsiella species [2]. Antimicrobial drug resistance to fluoroquinolone, cephalosporin, and carbapenem among Enterobacteriaceae has spread globally over the past few decades and become a pressing problem [3]. The dissemination of drug-resistant organisms is troublesome for clinicians when selecting empirical antibiotics. Patients with UTIs were historically administered broad-spectrum cephalosporin as the empirical therapy. Carbapenem is the definitive therapy for infections caused by extended-spectrum β-lactamases (ESBL)–producing bacteria. However, carbapenemsparing options are on the rise for mild infections with ESBL producers because its overuse is leading to the emergence of carbapenem-resistant organisms.
Recent studies have focused on the noninferiority of noncarbapenem therapy for the treatment of ESBL-producing Enterobacteriaceae infections to reduce carbapenem utilization [4-7]. A review article examined noncarbapenem β-lactam (cephamycin, cefepime, piperacillin/tazobactam, and newer β-lactam/β-lactamase inhibitors) therapy for ESBL-producing bacterial infections. The authors suggested that noncarbapenem could be considered in patients with mild to moderate low-inoculum infections [6]. A recent literature review summarized published articles regarding the treatment of ESBL-producing Enterobacteriaceae infections. Patients were divided into 3 groups: group 1, severe or nonsevere infections from high-risk sources and/or severely immunocompromised patients; group 2, nonsevere infections and intermediate-risk sources; and group 3, nonsevere infections and low-risk sources (Fig. 1). They concluded that carbapenem should be the choice of drug for the treatment of ESBL-producing Enterobacteriaceae in severe infections, whereas other antimicrobial agents could be considered for mild infections such as UTIs [7]. Thus, using noncarbapenem therapy for treating UTIs caused by ESBL-producing bacteria could be an effective way to prevent carbapenem overuse.
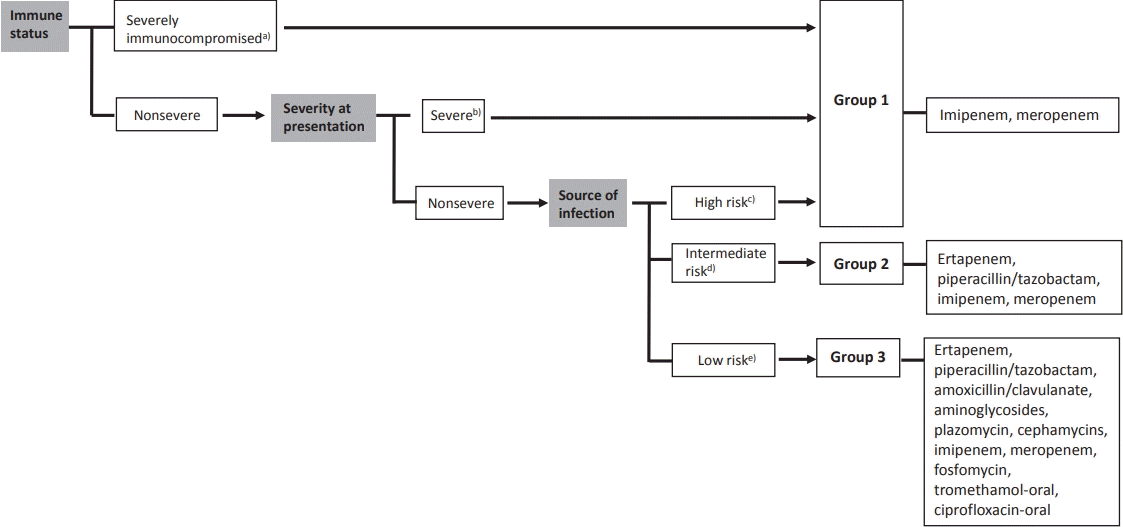
Classification of patients according to immune status, severity at presentation, source of infection, and treatment options for infections caused by extended-spectrum β-lactamase–producing Enterobacteriaceae by group.[7] a)Severely immunocompromised: neutropenia (<500/µL), leukemia, lymphoma, HIV infection with <200 CD4/µL, solid organ or hematopoietic stem cell transplantation, cytotoxic chemotherapy, steroids (15 mg of prednisone daily for >2 weeks); b)Severe: Pitt score ≥4, Acute Physiology and Chronic Health Evaluation II score > 10, intensive care unit admission, and presentation with severe sepsis or septic shock; c) High risk: high-inoculum infections, drainage impossible or inadequate (e.g., pneumonia, endocarditis, inadequately drained deepseated infections); d)Intermediate risk: not high or low risk; e)Low risk: urinary tract infection with no or a released obstruction.
Furthermore, children with vesicoureteral reflux (VUR) are at high risk for acute and recurrent pyelonephritis [8]. In patients with VUR, it is unknown whether carbapenem therapy can reduce the short-term recurrence. Therefore, a prospective study is needed to compare the treatment outcomes of carbapenemtreated and non–carbapenem-treated patients diagnosed with UTIs due to ESBL producers underlying VUR. To enable a careful conclusion, large samples and multivariate analysis are required.
If UTIs caused by ESBL-producing bacteria are alleviated through empirical noncarbapenem therapy, switching to carbapenem therapy is a difficult decision for clinicians. To solve this challenge and develop management guidelines, additional large-scale randomized controlled trials are required.
Notes
No potential conflicts of interest for this article are reported.
