Fluconazole prophylaxis against invasive candidiasis in very low and extremely low birth weight preterm neonates: a systematic review and meta-analysis
Article information
Abstract
Background
Evidence shows that fluconazole prophylaxis is an effective treatment against invasive fungal infections in preterm neonates, however, the most efficient schedule of fluconazole prophylaxis for the colonization and mortality of invasive candidiasis (IC) is unknown.
Purpose
This systematic review and meta-analysis aimed to assess the efficiency of different prophylactic fluconazole schedules in controlling IC colonization, infection, and mortality in very low birth weight (VLBW) and extremely low birth weight (ELBW) infants in neonatal intensive care units.
Methods
We searched the PubMed, Scopus, Embase, and Cochrane databases using the keywords “candida,” “invasive candidiasis,” “IC,” “fluconazole prophylaxis,” “preterm infants,” “very low birth weight infants,” “VLBW,” “extremely low birth weight,” and “ELBW.”
Results
Mortality was significantly decreased in a meta-analysis of studies using different fluconazole prophylaxis regimens. The meta-analysis also indicated a significant decrease in the incidence of IC-associated mortality in ELBW infants using the same fluconazole prophylaxis schedules.
Conclusion
Future studies should explore the effectiveness of other different fluconazole prophylaxis schedules on IC colonization, infection, and mortality.
Key message
· Mortality is decreased significantly in meta-analysis of studies in different regimen of fluconazole prophylaxis.
· Significant decrease was seen in incidence of invasive candidiasis-associated mortality in extremely low birth weight infants in same schedules of prophylaxis.
· More studies required to relief the concerns.
Graphical abstract. In a systematic review and meta-analysis, the efficiency of different prophylactic fluconazole schedules in controlling invasive candidiasis (IC) colonization, infection, and mortality were assessed in very low birth weight (VLBW) and extremely low birth weight (ELBW) infants in neonatal intensive care units. There was a significant decrease in the incidence of IC-associated mortality in ELBW infants using the same fluconazole prophylaxis schedules. RR, risk ratio.
Introduction
Invasive candidiasis (IC) in very low birth weight (VLBW) infants can be fatal and often results in neurodevelopment impairment. IC as a fungal infection in the blood and other sterile body liquids is a frequent cause of mortality in VLBW infants. Despite antifungal treatment, patients with IC have a mortality rate of 20%–60%, while surviving babies may develop neurodevelopmental impairments [1,2].
The extensive involvement of IC in the neonatal intensive care unit (NICU) is related to immaturity of the immune system and other risk factors including prematurity, intratracheal intubation or catheterization, surgery, prevention of infection, and the administration of antibiotics and corticosteroids. Fungal infection in neonates often originates from Candida albicans and on recently Candida parapsilosis [3-7]. Successive control of candidiasis in infants requires proper and effective antifungal and supportive treatments to prevent IC [3,4]. A progressive fluconazole prophylaxis showed efficiency, immunity, and prolonged positive neurodevelopmental results in NICU infants in an evaluation at 2 years of age, with more than 15% of the risk consisting of problems such as profound bilateral hearing loss, severe cognitive delay, and severe cerebral palsy [8-14]. In addition, antifungal prophylaxis reduces the incidence of mortality due to invasive fungal infection in VLBW infants [15].
A meta-analysis of 5 randomized clinical trials (RCTs) demonstrated that prophylaxis consisting of different 42-day fluconazole treatment schedules effectively controlled mortality compared to 28-day treatment [16]. Several studies compared different fluconazole doses and administration schedules to identify the best treatment option [10,17,18]; although it remains unknown which schedule and treatment dose is most efficient for controlling colonization (implantation and growth of a microorganism on a host), mortality (as the quality being subject to death), and IC in VLBW infants [19].
The current systematic review and meta-analysis aimed to evaluate the different regimens to identify the most efficient fluconazole prophylaxis schedule against IC in VLBW infants.
Methods
1. Search strategy
This systematic review and meta-analysis was conducted according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines [20]. Two investigators independently searched the PubMed, Scopus, Embase, and Cochrane databases for all eligible studies. Forward citation tracking was also performed to identify additional relevant studies. The keywords used in the search were “invasive candidiasis,” “candida,” “fluconazole prophylaxis,” “preterm infants,” “very low birth weight infants,” “VLBW,” “extremely low birth weight,” and “ELBW.” Two researchers independently searched for all relevant English articles published between 2001 and January 2018 and then performed forward citation tracking. The selected studies were RCTs and cohort studies with historical controls that explored the effect of prophylaxis with fluconazole in first 24 hours of life in VLBW infants on the incidence of IC colonization and mortality rates versus placebo or without fluconazole prophylaxis. Methodological quality was assessed using standardized methods, for instance, the Newcastle-Ottawa Scale was used to assess cohort studies [21]. The results were compared, and any questions or discrepancies were resolved through iteration and consensus.
2. Data extraction
The following data were extracted from the retrieved studies: baseline characteristics (authors, year of publication, number of patients, birth weight, and antifungal therapy characteristics) and outcomes of interest (incidence of proven IC, colonization, overall mortality, and IC-related mortality). A total of 174 articles were selected: 43 from PubMed, 32 from Scopus, 15 from Embase, 21 from Cochrane, and 63 from the manual search. Among the 174 articles, 84 were duplicated and 90 remained. Of them, 74 were excluded, including 29 reviews and expert commentary, 9 epidemiology of IC articles, 7 case reports, 5 case-control articles, 3 drug schedules and hygiene in NICU, 9 studies of pharmacokinetics of antifungal drugs, and 12 with unrelated titles from original articles describing antifungal prophylaxis use in VLBW infants. Sixteen studies were eligible for comparative evaluation (Fig. 1). As mentioned in Fig. 1, 3 studies that postponed starting prophylaxis more than 24 hours after birth were excluded. The primary outcome of this meta-analysis was mortality, while the secondary outcomes were colonization and IC.
3. Included studies
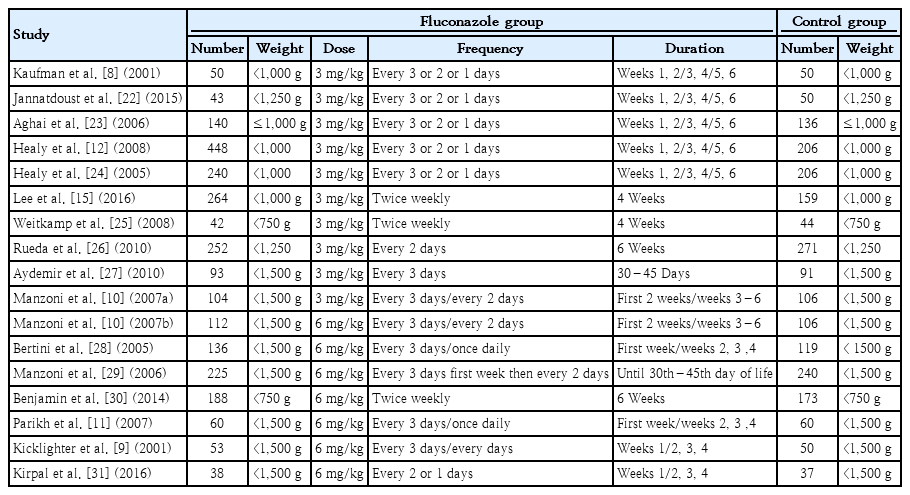
For assessing the incidence of IC and mortality in VLBW and extremely low birth weight (ELBW) infants, fluconazole prophylaxis regimens used in 16 studies were reviewed [8-12,15, 22-31]. The study characteristics are mentioned in Table 1. All schedules consisted of 3- or 6-mg/kg fluconazole mostly for 4–6 weeks in 7 schedules for infants with a birth weight less than 1,500 g.
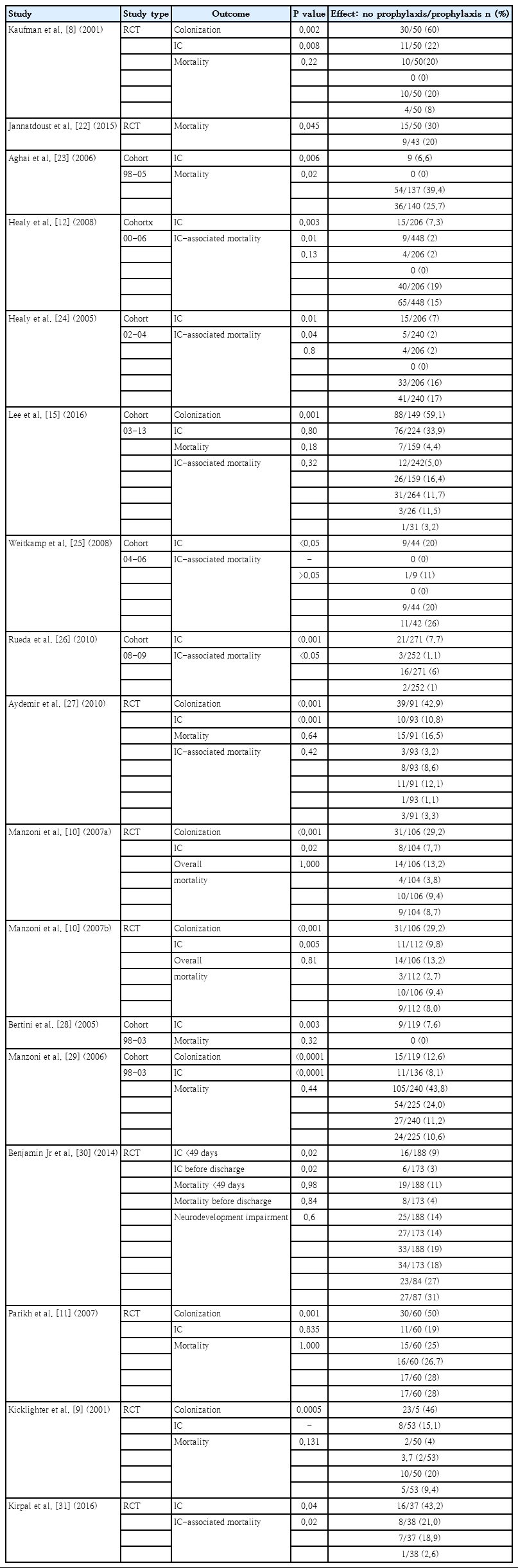
IC and/or mortality with or without colonization and IC-associated mortality were evaluated in 4,486 subjects in 9 RCTs and 8 cohort studies with 1,358 and 3,128 subjects, respectively, to compare the effect of prophylaxis with fluconazole and treatment with placebo or no prophylaxis in ELBW and VLBW infants. Table 2 shows the outcomes in addition the author’s name and year of the publication of every study. The meta-analysis included 4 groups of 2 or 3 studies each that had same study protocol, dose, frequency, and duration.
4. Statistical analyses
In every study, the risk ratios (RRs) for colonization, IC, IC-associated mortality, and mortality were determined by meta-analysis and the pooled RR was calculated to assess outcomes. The I2 statistics and Cochrane Q were derived to estimate the fraction of variability between study RRs due to heterogeneity rather than chance. I2 values of 25–75 and I2 >75 were considered middle and high heterogeneity, respectively. In cases of heterogeneity, the random-effects model was used to determine the overall effect size [32]. Egger regression test and a funnel plot were used to assess publication bias. Statistical analyses were performed using comprehensive meta-analysis ver. 2.0 software (Biostat Inc., Englewood, CO, USA), and P values <0.05 were considered significant.
Results
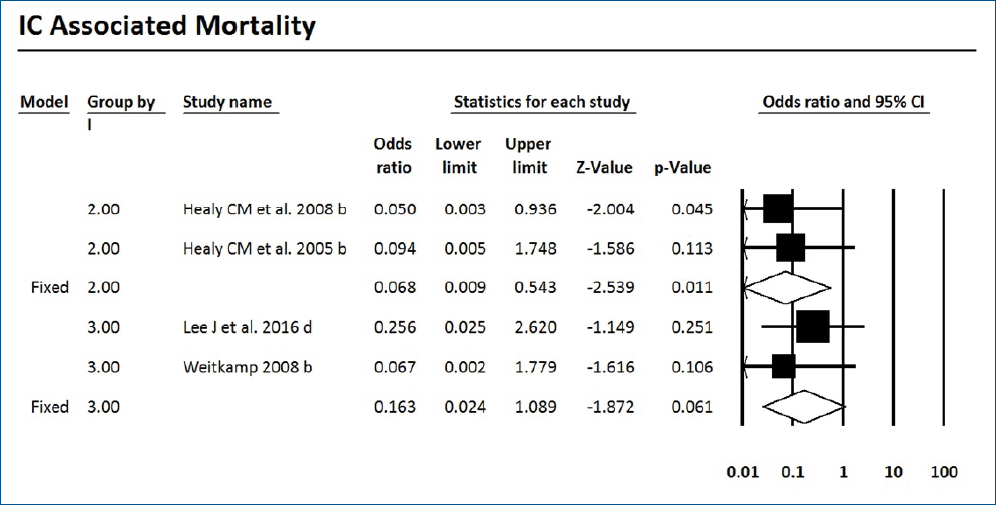
1. IC-associated mortality
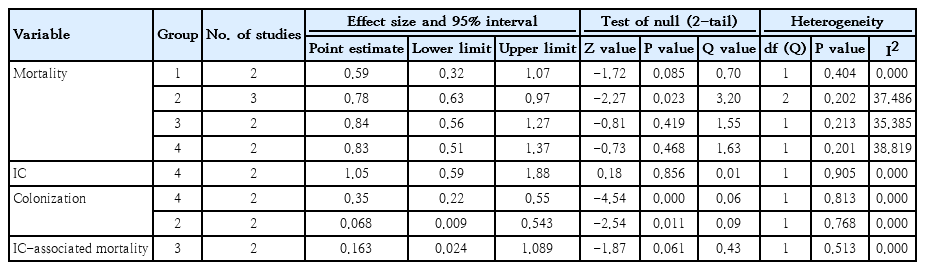
Mortality with or without IC was surveyed of the 7 RCTs and 9 cohort studies. Six studies showed significantly decreased mortality and/or IC-associated mortality in the prophylaxis groups. Rueda et al. [26] showed less IC-associated mortality in relation to all-cause death by prophylaxis year (P<0.05), while most deaths occurred in infants less than 1,000 g and neonates born at less than 31-week gestation. Kirpal et al. [31] reported that IC-associated mortality was significantly decreased with prophylaxis use in infants (Table 2). Regardless of schedule, the current meta-analysis showed that prophylaxis with fluconazole can significantly decrease the IC-associated mortality of VLBW infants weighing ≤1,000 g (P=0.011) (RR, 0.068; 95% confidence interval [CI], 0.009–0.543) (Figs. 2, 3) with different regimens and the administration of 3-mg fluconazole every 3 days in weeks 1 and 2, then every 2 days in weeks 3 and 4, and every day in weeks 5 and 6 decreased mortality rates significantly (P=0.023) (RR, 0.780; 95% CI, 0.629–0.966). The heterogeneity was not significant in these 2 outcomes as shown in Table 3 (group 2 for mortality: Q=3.20, degrees of freedom [df]=2, P=0.202, I2=37.486; group 2 for IC-associated mortality: Q=0.09, df=1, P=0.768, I2<0.001).
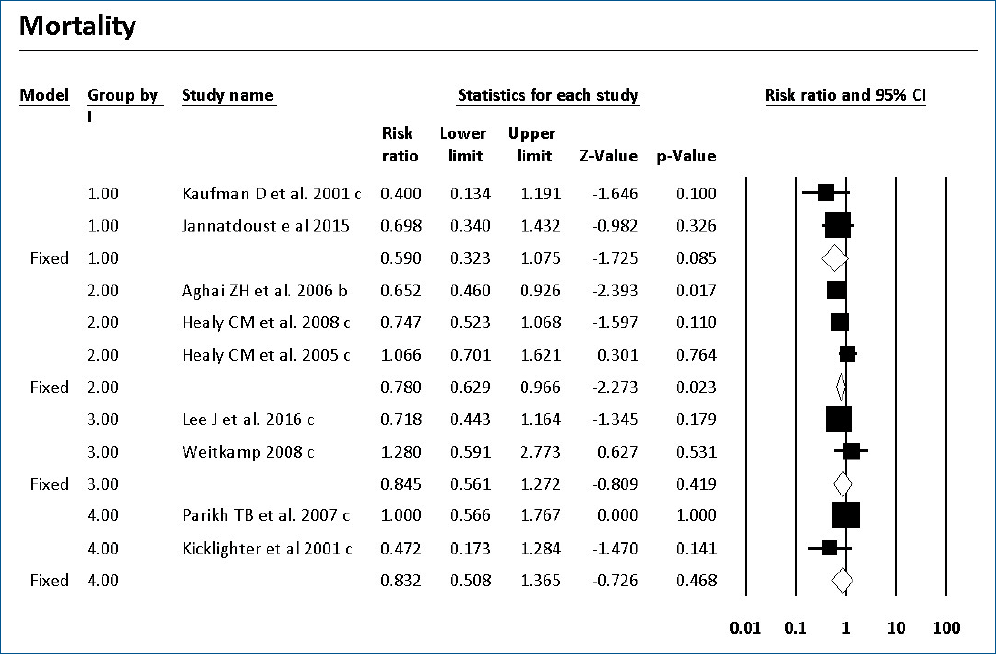
Forest plot of risk ratio between prophylaxis with fluconazole and no prophylaxis for mortality in very low birth weight infants. CI, confidence interval.
2. Colonization and IC
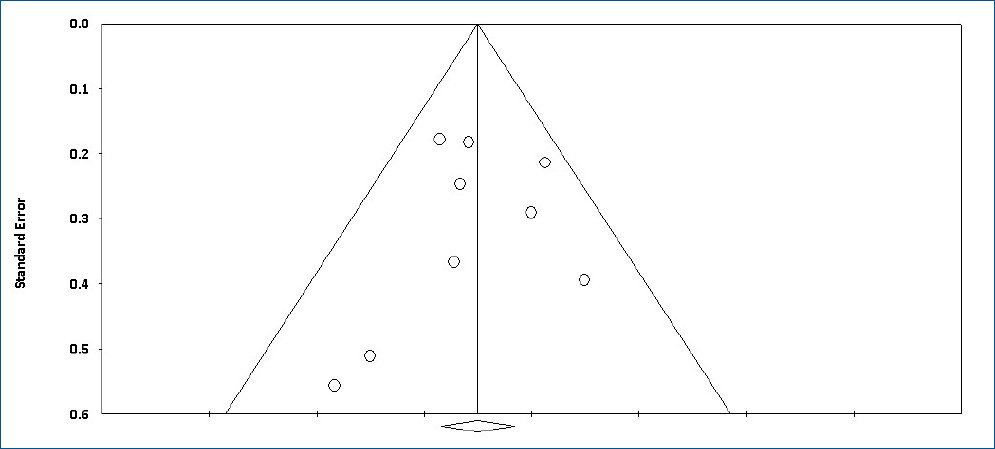
The colonization of Candida spp. in different locations (endotracheal secretion, nasopharynx, periumbilical region, perineum, gastric aspirate, skin) was evaluated in 7 studies (Table 2). All showed significantly decreased colonization, and the meta-analysis showed that 6 mg fluconazole administered every 3 days in week 1 and every day in the following 3 weeks can prevent colonization in VLBW infants (Fig. 4) (P<001; RR, 0.350; 95% CI, 0.223–0.551). Additionally, IC was decreased significantly on day 10, but 3 studies in the prophylaxis group versus placebo or the no prophylaxis group reported data in different groups, most of which were not suitable for analysis or the assessed schedules ineffectively prevented IC in the meta-analysis (Fig. 5). The heterogeneity was not significant in these outcomes as depicted in Table 3 (group 4 for colonization: Q=0.06, df=1, P=0.813, I2<0.001; group 4 for IC: Q=0.01, df=1, P=0.813, I2<0.001). The funnel plot of standard error by log RR is shown in Fig. 6. The results of Egger regression test were t=0.38, df=7.0, and P=0.71.

Forest plot of risk ratio between prophylaxis with fluconazole and no prophylaxis for colonization in very low birth weight infants. CI, confidence interval.
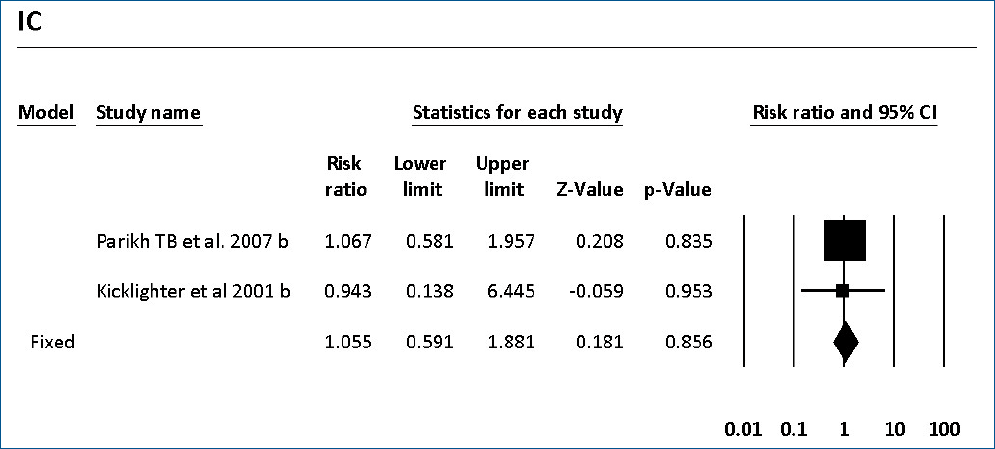
Forest plot of risk ratio between prophylaxis with fluconazole and no prophylaxis for invasive candidiasis in very low birth weight infants. CI, confidence interval.
Discussion
Fungal invasion is a severe infection in VLBW infants that can result in mortality or otherwise affect quality of life. Prophylaxis with fluconazole both decreases mortality and decreases the incidence of neurodevelopmental impairments. Fluconazole prophylaxis is currently recommended for NICUadmitted infants with a ≥5% risk of an incidence of IC by many continental neonatology associations [33-37].
The current meta-analysis showed that prophylaxis with fluconazole administered according to 1 of 7 surveyed schedules compared with no prophylaxis or placebo can significantly decrease the overall mortality rates of ELBW infants less than 1,000 g (RR, 0.780; 95% CI, 0.629–0.966). This effect was driven by the studies that used a dose of 3-mg/kg fluconazole every 3 days in weeks 1 and 2, every 2 days in weeks 3 and 4, and every day in weeks 5 and 6. IC-associated mortality also decreased significantly with fluconazole prophylaxis whenever colonization prevented with a dose of 6-mg/kg fluconazole every 3 days in week 1 and every day in the following 3 weeks in 2 RCTs of VLBW infants weighing <1,500 g.
Some studies reported that prophylaxis with fluconazole can decrease IC and/or colonization without significantly affecting mortality [38-40].
A candidiasis management guideline recommended intravenous or oral fluconazole prophylaxis in nurseries with high rates (>10%) of IC using 3–6 mg/kg twice weekly for 6 weeks in neonates with birth weights <1,000 g. Whenever this schedule with 3-mg/kg fluconazole assessed for preventing IC-associated mortality in current study had no significant effect (P=0.06) [36], 3-mg/kg fluconazole every 3 days in weeks 1 and 2, every 2 days in weeks 3 and 4, and every day in weeks 5 and 6 prevented IC-associated mortality (P=0.011). The differences in results between these studies and the current study can be attributed to differences in the studies assessed, the proposed endpoints, and the populations under study.
There were some limitations to the current study. First, the differences in study duration and medication administration might have created heterogeneity in the outcomes. Second, the endpoints differed between the current study and surveyed studies. Third, no antifungals other than fluconazole were investigated. Fourth, the subjects’ gestational ages and birth weights were not evaluated in this study. And finally, the following potential issues with the introduction of fluconazole prophylaxis to NICU patients should be considered: drug side effects, changes in susceptibility of antifungal agents, changes in pathogenic strains, and emergence of resistant strains. Despite more limitations in similar studies that should be considered when evaluating and generalizing their results, the high incidence of IC-associated mortality and complications in premature infants even after treatment resulted in the recommendation of preventive measures, including the use of prophylactic fluconazole [16].
In conclusion, the study showed the effectiveness of 2 schedules of fluconazole prophylaxis with a significant reduction in the incidence of colonization by Candida spp., IC-associated mortality, and total mortality. This effect was further driven by studies that used 3-mg/kg fluconazole every 3 days in weeks 1 and 2, every 2 days in weeks 3 and 4, and every day in weeks 5 and 6. Concerns about the use of fluconazole prophylaxis included side effects, induction of resistance, and effect on mortality and IC.
Notes
No potential conflict of interest relevant to this article was reported.