Association between maternal smoking during pregnancy and risk of bone fractures in offspring: a systematic review and meta-analysis
Article information
Abstract
This study aimed to investigate the effect of maternal smoking during pregnancy (MSDP) on the risk of bone fractures in the offspring through a systematic review and meta-analysis. The PubMed, Web of Science, and Scopus databases were systematically searched for relevant articles published through July 2019. According to heterogeneity, the pooled risk ratio (RR) and odds ratio (OR) and their corresponding 95% confidence interval (CI) were obtained using fixed or random effects models. The heterogeneity and quality of the included studies were assessed by the I-squared (I2) statistic and the Newcastle-Ottawa scale, respectively. Sensitivity analyses were performed to test the effect of MSDP misclassification on the results. The review of 842 search records yielded 5 studies including 8,746 mother-child pairs that were included in the meta-analysis. Pooling adjusted effect measures showed that MSDP was not associated with a later risk of bone fractures in the offspring (pooled RR, 1.15; 95% CI, 0.84–1.58; I2=66.8%; P=0.049). After the adjustment for misclassification, MSDP may be associated with a 27% increased risk of bone fracture (pooled OR, 1.27; 95% CI, 1.00–1.62; I2=0%; P=0.537). After the adjustment for misclassification, MSDP is associated with an increased risk of bone fractures among children whose mothers smoked during pregnancy.
Key message
Question: What is the effect of maternal smoking during pregnancy (MSDP) on the risk of bone fractures in the offspring?
Finding: After the adjustment for misclassification, MSDP may be associated with a 27% increased risk of bone fracture in the offspring (pooled odds ratio, 1.27; 95% confidence interval, 1.00–1.62; I2=0%; P=0.537).
Meaning: Preventive measures and health education programs should be designed and implemented to encourage women to stop smoking, especially during.
Graphical abstract. The effect of maternal smoking during pregnancy (MSDP) on the risk of bone fractures in offspring after the adjustment for MSDP misclassification
Introduction
Bone fracture is one of the most common injuries in children. An estimated 27%–50% of children suffer from bone fractures before 18 years of age [1,2]. It has been suggested that even children without a history of bone diseases may experience frequent fractures in childhood and adolescence [2]. Unbiased and larger epidemiological studies are required to confirm the factors associated with bone fracture occurrence in children and youth. Studies of the etiology of bone fractures suggest that some characteristics of children [3-10] and parents [11-15] may be associated with an increased risk of bone fractures in children and youth.
Maternal smoking during pregnancy (MSDP) is one of the parental characteristics that was recently suggested as a risk factor of bone fractures in offspring [12]; however, its role as a risk factor was not confirmed in all studies. For example, it was a risk factor in one study [16] but a nonsignificant negative factor between 2 aforementioned factors in another study [17]. Evidence of the association between MSDP bone fractures in offspring was primarily derived from observational studies, which are prone to many possible sources of bias. The results of studies concerning the association between MSDP and bone fractures may be prone to recall bias. In other words, smoking during pregnancy is recalled by mothers after having given birth [17] or even later [2], and mothers of children with bone fractures are likely to remember smoking during pregnancy differently than mothers of fracture-free controls.
Considering the above issues, the present study aimed to systemically review and meta-analyze potential studies concerning the association between MSDP and bone fractures in offspring and adjust for the effect of MSDP misclassification on the results.
Methods
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [18].
1. Search strategy
The PubMed, Scopus, and Web of Sciences databases were searched up to July 2019. The search strategy was developed by combining keywords including “bone fractures and maternal smoking and pregnancy and children.” The details of the search strategy for each database are presented in Supplementary material 1. Moreover, the reference lists of the included articles were also manually screened to identify relevant studies that the search strategy failed to retrieve.
2. Study selection
Studies identified in the initial search were imported into bibliographic citation management software (Endnote X6; Clarivate Analytics, Philadelphia, PA, USA) for screening and duplicate checking. After duplications were discarded, the titles and abstracts of the identified studies were reviewed independently by 2 authors (EA and KM). In cases of disagreement, the 2 authors discussed the article and reached consensus.
3. Eligibility criteria
In the next stage, the full texts of eligible original studies were reviewed to obtain more details. All original observational studies that evaluated the association between MSDP and bone fracture in the offspring and met following criteria were considered for this systematic review and meta-analysis: observational studies that reported relative measures and confidence intervals (CIs); and provided crude data for estimating the aforementioned association. Animal and lab studies, case reports, reviews, meeting abstracts, correspondence, and editorials were excluded.
4. Data extraction
The data extraction was performed by 2 independent authors (EA and KM) using a standard form in Excel software and included the following: first author, year of publication, country, study design, age and sex of children who experienced bone fractures, sample size, number of bone fracture cases, diagnostic methods of MSDP and bone fractures, type of relative measures (95% CI), and adjusted covariates.
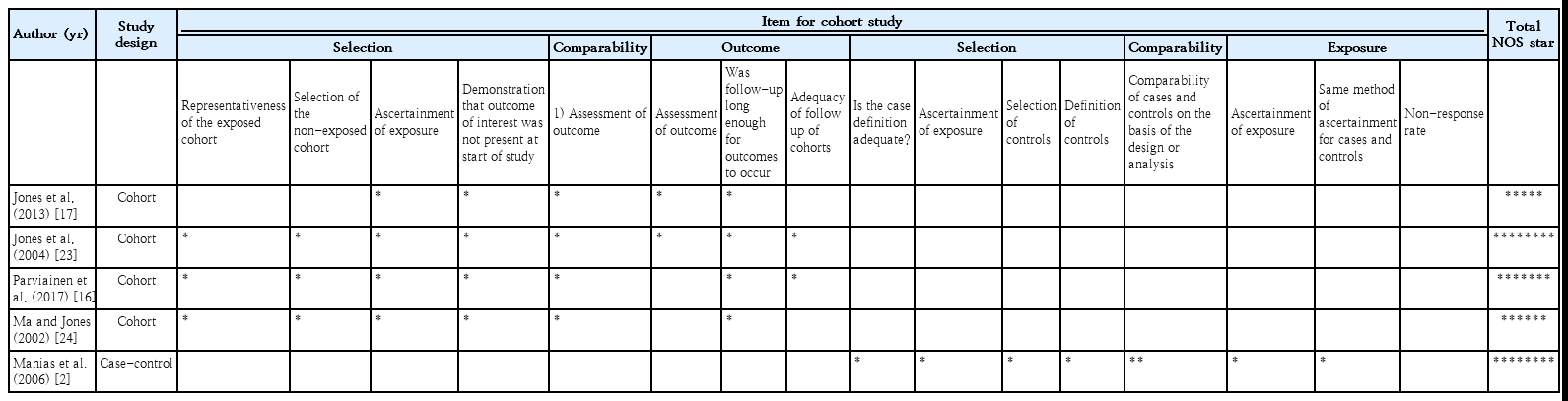
The quality assessment was performed using the Newcastle-Ottawa Scale (NOS) [19]. This scale criticized cohort and case control studies according to 3 domains including study group selection (4 items), study group comparability (2 items), and ex posure and outcome measurements (3 items); those who received a NOS score of at least 6 were considered of high quality. Notably, only one author (KM) conducted a quality assessment of the included studies.
5. Statistical analysis
The relative measures were risk ratio (RR) and odds ratio (OR) with 95% CI. Heterogeneity among the included studies was assessed using the I2 statistic and Cochran Q test, on which values higher than 50% and P<0.05 imply substantial heterogeneity [20]. Regardless of interstudy heterogeneity, the pooled relative measure were calculated using both a fixed effect model (inverse variance) and a random effects model (I–V heterogeneity) to assess the impact of small study effects on the results. No attempt was made to assess potential publication bias when fewer than 10 studies were included [21]. Values of P<0.05 were considered significant.
6. Sensitivity analysis
In the included studies, information about MSDP were gathered based on mothers’ recall; thus, the information may be subject to misclassification bias. In other words, mothers may report smoking without actual exposure or vice versa, the sensitivity and specificity of recall is less than 100%. Here we used a Bayesian bias model to test the effect of the potential MSDP misclassification on the results. The details of the sensitivity analysis using the Bayesian model are presented in Supplementary material 2.
Results
1. Study characteristics
Fig. 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the review process. The initial search identified 842 articles. After the removal of duplicates using Endnote X6 (n=43), the titles and abstracts of 799 articles were screened and 7 eligible articles were ultimately identified [12,16,17,22-25]. The full-text review of those 7 articles revealed that 3 did not meet the eligibility criteria [12,22,25]. Review of the reference lists of the eligible articles identified another article [2]; finally, 5 articles [2,16,17,23,24] were included in the systematic review and meta-analysis.
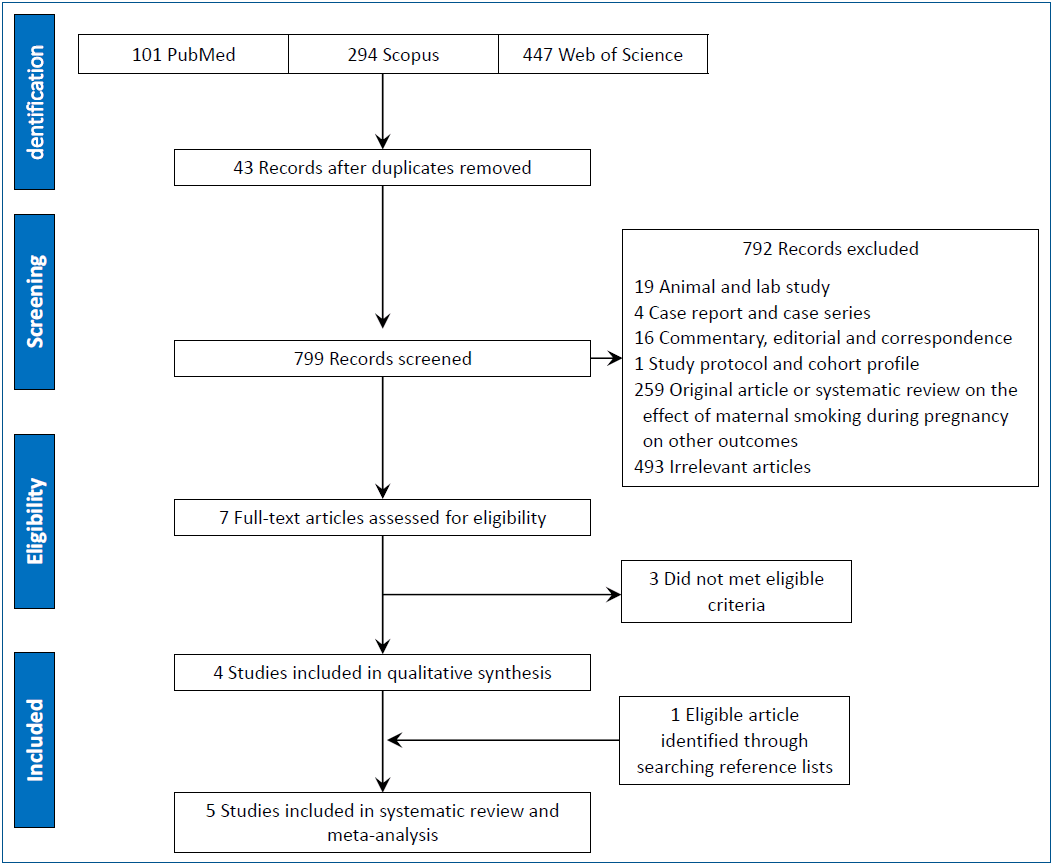
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the study selection process for a systematic review and meta-analysis of the association between maternal smoking during pregnancy and the risk of fractures.
Table 1 displays the characteristics of the included studies. The included studies were conducted in Australia (2 studies) [17,24], the UK (1 study) [2], New Zealand (1 study), and Finland (1 study) [16]. Most of the included studies (n=4) used a cohort study design that involved a total of 8,596 children, including 901 with at least one bone fracture before age 18 [16,17,23,24]. The other included study had a case control design [2] and involved 100 cases and 50 controls. Boys more often suffered from fractures than girls. In all included studies, the ascertainment of smoking during pregnancy was based on mothers’ recall.
2. Quality of included studies
Table 2 shows results of the risk of bias assessment using the Newcastle-Ottawa Scale (NOS). According to the NOS, all of the included studies received a score≥6 except for the study by Jones et al. [17], which had a NOS score of 5.
3. Effect of MSDP on bone fracture
Figs. 2–4 show the results of the fixed and random effects meta-analysis of the association between MSDP and bone fractures. According to the fixed effect model, the overall crude OR (95% CI) was 1.40 (1.06–1.85), I2=5.8%, P=0.364 (Fig. 2). According to the random effects model, the overall adjusted RR (95% CI) of the risk of bone fractures in children whose mothers smoked during pregnancy was 1.15 (0.84–1.58), I2= 66.8%, P=0.049 (Fig. 3). In 2 approaches (deterministic and probabilistic), the fixed effect meta-analysis of overall OR after the correction for misclassification shows that MSDP increases the odds of bone fractures by 27% (I2=0.0%, P=0.537, I2= 0.0%, P=0.548, respectively) (Fig. 4).
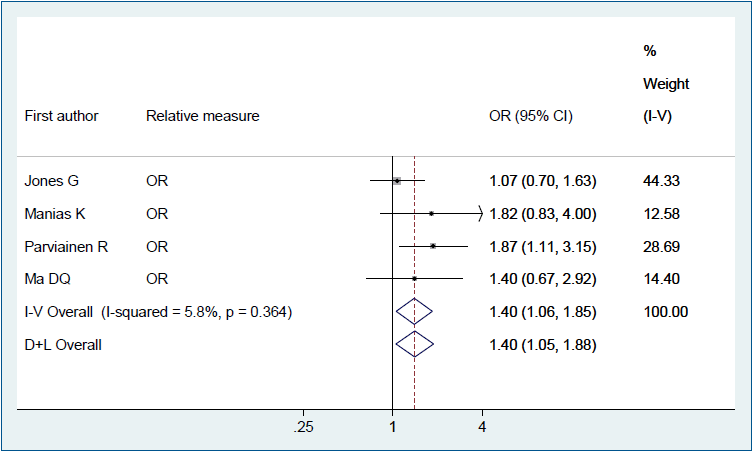
Forest plot of 4 studies showing the crude effect of maternal smoking during pregnancy on the risk of bone fractures in offspring through a fixed effect model. CI, confidence interval; D+L, DerSimonian and Laird; I-V, inverse variance; OR, odds ratio.
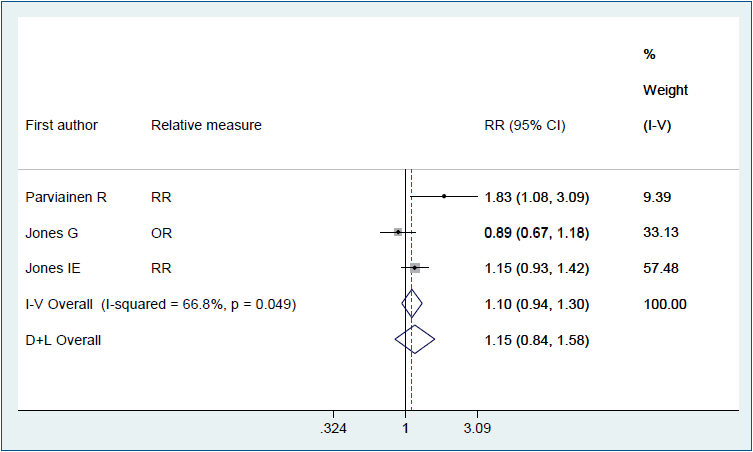
Forest plot of 3 studies showing the adjusted effect of maternal smoking during pregnancy on the risk of bone fractures in offspring through a random effects model. CI, confidence interval; D+L, DerSimonian and Laird; I-V, inverse variance; OR, odds ratio; RR: risk ratio.

Forest plot of 4 studies showing the crude effect of MSDP on the risk of bone fractures in offspring after the adjustment for MSDP misclassification through fixed-effect model: (A) deterministic sensitivity analysis; and (B) probabilistic sensitivity analysis. MSDP, maternal smoking during pregnancy; CI, confidence interval; D+L, DerSimonian and Laird; I-V, inverse variance; OR, odds ratio.
Discussion
This study aimed to determine the association between MSDP and risk of bone fractures in offspring using a systematic review and meta-analysis. The results demonstrated that MSDP is associated with an increased risk of bone fractures; i.e., children whose mothers smoke during pregnancy are 15% more likely to suffer from bone fractures than children whose mothers do not smoke during pregnancy (overall RR, 1.15; 95% CI, 0.84–1.58); however, this increased risk was not statistically significant. However, after the correction for MSDP misclassification, we observed a significant association between MSDP and bone fractures; thus, MSDP may be associated with a 1.27-fold increased risk of bone fractures among children of smoking mothers (overall RR, 1.27; 95% CI, 1.00–1.62; I2=0%; P= 0.537). The findings of studies concerning the asso ciation between MSDP and the risk of bone fractures in offspring are conflicting.
The inconsistency noted across the included studies may have been due to several reasons. First, although the resulting effect measures tended to shrink toward null after the correction of MSDP misclassification, the degrees of recall bias across the included studies were relatively different. Second, among the included studies, covariate adjustments were not performed in the same manner, and it seems that they did not attempt to adjust for all potential confounders. For example, in the study of Manias et al. [2], in addition to MSDP, fizzy drink intake, milk intake bone area, bone mineral content (BMC), bone mineral density (BMD), height, weight z scores, physical activity, and diagnosis of asthma were included in the multivariate analyses. In the Parviainen et al. [16] study, sex, childhood rheumatism, asthma, body mass index, family’s socioeconomic status, and maternal age were potential confounders. Third, the quality of the studies of the association between MSDP and bone fractures showed a high degree of heterogeneity.
The mechanism of the effect of maternal smoking on children’s skeletal development is unclear. However, cigarette smoke contains thousands of harmful substances that can directly disrupt the formation of a growing skeleton. Evidence suggests that maternal smoking can reduce calcium absorption and cause placental dysfunction. Nutrient deficiency impairs fetal bone development [17,26,27]. In a prospective birth cohort study that aimed to evaluate the effect of parental smoking during pregnancy on the bone mass of 7,121 children at 10 years of age, maternal smoking was associated with an increased risk of total body less head, spine BMC, bone area, and BMD in girls; however, the relationship was not significant for boys [25].) A large birth cohort study of 6,718 children in Northern Finland showed that MSDP was directly associated with an increased risk of in-hospital–treated fractures at pre-school age (RR, 1.83fold; 95% CI, 1.06–3.02; P=0.022). This study also suggested an increased risk of bone fractures due to disordered fetal bone development as a result of maternal smoking [16]. Furthermore, in some studies, maternal smoking has been recognized as a limiting factor for fetal growth [28], which can result in low birth weight, a risk factor for a lower BMC in childhood and adulthood.
A number of other potential mechanisms have been suggested for the harmful effect of MSDP, including impaired placental size and function, a low maternal blood sugar, maternal diet disorder, and low volume of breast milk [29-31]. Smoking has the greatest effect on placental function. Various studies have reported defects in placental function and size along with changes in endothelial function and epidermal growth factor in smoking mothers [32,33]. Jones et al. [22] reported a decrease in the placental weight of smoking mothers; after the adjustment for placental weight, the effect of smoking was not significant, which shows that this is an intermediate variable regarding the harmful effect of MSDP. Studies have suggested that different compounds in cigarettes can lead to impaired bone turnover. As a result, smoking causes the formation of bones that are prone to fractures. Clinically, these adverse effects are due to a significant loss of BMD related to smoking, which can vary depending on the degree of cigarette exposure [27].
The study also had some strengths and limitations. First, to the best our knowledge, this is the first meta-analysis to investigate the association between MSDP and bone fracture in offspring. Second, most of the final studies included in the meta-analysis were cohort studies, the strongest observational study type, with high sample sizes and low risk of bias. And third, we handled misclassification bias using a Bayesian bias model to evaluate the effect of the potential MSDP misclassification on the findings. This study also has several limitations. First, a total of 5 studies were included in the meta-analysis, which makes it impossible to determine the true effect of publication bias on the results. Second, this study may be subject to some degree of selection bias due to missing potential studies.
In conclusion, the resulting associations from these observational studies should be interpreted with caution due to potential biases such as misclassification bias. After accounting for misclassification bias, this systematic review and meta-analysis demonstrated that MSDP may be associated with an increased risk of bone fractures among children whose mothers smoked during pregnancy. Therefore, primary prevention measures and health education programs should be designed and implemented to encourage women to stop smoking, especially during pregnancy.
Notes
No potential conflict of interest relevant to this article was reported.
Supplementary materials
Supplementary materials 1-2 can be found via https://doi.org/10.3345/cep.2019.01466.Supplement.
Search strategy.
Bayesian analysis.