Characteristics and prognosis of hepatic cytomegalovirus infection in children: 10 years of experience at a university hospital in Korea
Article information
Abstract
Purpose
Studies on cytomegalovirus (CMV) infections in immunocompetent children are lacking, and minimal information is available in the medical literature on hepatic manifestations and complications of CMV. The aims of this study were to evaluate the clinical characteristics, laboratory data, and prognosis of children with CMV hepatitis, and to investigate its prevalence at a single medical center in Korea over a 10-year period.
Methods
One hundred thirty-two children diagnosed with CMV infection based on specific markers (anti-CMV IgM, CMV polymerase chain reaction in blood and urine, or CMV culture of urine) were included in the study. Clinical and biochemical characteristics, immunological markers, and outcomes of hepatic CMV infection were determined.
Results
The median age of patients (n=132) was 8.5 months (range, 14 days–11.3 years). Peak total bilirubin and alanine aminotransferase levels in serum ranged from 0.11–21.97 mg/dL, and 5–1,517 IU/L, respectively. Alanine aminotransferase remained elevated from 2–48 weeks. Jaundice was the most common clinical feature of hepatic CMV infection during infancy. The hematologic findings revealed anemia, leukocytosis, and monocytosis in CMV-infected patients. All participants recovered without administration of ganciclovir.
Conclusion
In children with CMV hepatitis, fever was the most common symptom at presentation, and jaundice was the most common clinical feature of hepatic CMV infection in infants younger than 3 months of age. Hepatic CMV infection in immunocompetent children is often a self-limited illness that does not require antiviral therapy, as most patients in this study had favorable outcomes.
Introduction
Human cytomegalovirus (CMV) was first isolated by 3 different groups of investigators, Rowe and colleagues, Weller and colleagues, and Smith, simultaneously in 19561). Since then, CMV has been recognized as the most common congenital viral infection in humans, affecting 30,000 to 40,000 infants each year in the United States. The incidence of congenitally acquired CMV ranges from 0.5% to 2.2% of all live births in the developed world. Congenital CMV infection is the leading nongenetic cause of sensorineural deafness and mental retardation in children12).
CMV is transmitted by close contact between individuals, through urine contamination, oropharyngeal secretions, tears, semen, cervical secretions, and breast milk3). Infants may acquire CMV infection from their mothers through intrauterine infection (congenital infection), through contact with infected genital secretions during passage through the birth canal (perinatal infection), or postpartum through breastfeeding (postnatal infection)4). Although 90% of congenitally infected infants with CMV are asymptomatic at birth, approximately 10% develop cytomegalic inclusion diseases. In postnatal CMV infection, a majority of infants are also asymptomatic, but frequently show hepatic involvement5). A few infected infants have diseases such as pneumonitis and mononucleosis6). CMV hepatitis is relatively common in young age, especially in early infancy, and is associated with cholestasis during this period. CMV infection during infancy might be dangerous since it can result in cirrhosis and can occasionally be lethal78). However, there is little information on the hepatic features of CMV infection in infancy. Beyond infancy, 50%–60% of individuals younger than 25–30 years of age are infected with CMV. The prognosis of CMV hepatitis has been known to be good in immunocompetent infants and healthy individuals, but only a few studies have described the clinical course of CMV hepatitis9).
The aims of this study were to evaluate the clinical characteristics, laboratory data, and prognosis of immunocompetent children with CMV infection, particularly their hepatic features, and to investigate its prevalence at a single medical center of a University hospital in Korea, over a 10-year period.
Materials and methods
1. Patients
This study was conducted at the Department of Pediatrics, Bundang CHA Medical Center, CHA University. During a 10-year period (January 2005–July 2015), all children with hepatic dysfunction that correlated with documented CMV infection were retrospectively reviewed and included in the study. The study design was approved by the Institutional Review Board of Bundang CHA Medical Center (approval number: 2015-10-166).
The authors identified patients with hepatic CMV infection and reviewed their medical records for details on clinical presentation and laboratory data.
Inclusion criteria were: (1) age between 0 months and 15 years; and (2) diagnosis of hepatic CMV based on the presence of specific IgM CMV antibody titers in serum, detection of CMV PCR in blood, and/or detection of CMV cultures or PCR in urine.
The evaluation of IgM antibody titers against CMV was performed using chemiluminescence micro particle immunoassay. Quantitative CMV PCR was performed using CMV Quant-kit for the quantitative detection of CMV-DNA in blood and urine samples (GC Labs, Yongini, Korea).
Exclusion criteria were: (1) congenital and acquired immunodeficiency; (2) metabolic liver disorders, other autoimmune, genetic, or toxic liver diseases; (3) other viral hepatitis infections; (4) documented respiratory or stool virus infections; (5) documented bacterial infections such as sepsis and urinary tract infections; (6) abdominal and hepatic surgical causes; or (7) Kawasaki disease, muscular diseases, and systemic disorders.
We conducted CMV PCR and/or specific IgM CMV antibody titers in 1,238 patients. Of these, 160 patients were detected to serum specific IgM or CMV PCR in blood or urine. Of these, 132 were included in the study because 28 patients were eligible for exclusion criteria.
2. Statistical analysis
Data from each infant were entered into an electronic database and analyzed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). The chi-square test was used to analyze the categorical data, along with Fisher exact test when applicable. The t test was used for continuous data.
Results
1. Patient groups
We identified 132 patients with hepatic CMV in the present study. There was a ratio of 74 boys (56%) to 58 girls (44%). The median age at presentation was 8.5 months (14 days–11.3 years). There were 73 infants (55.3%) younger than 3 months old, and 59 children (44.7 %) were older than 3 months. Twenty patients (15.2%) were born preterm at less than 37 weeks. Sixty-three patients (47.7%) were born by vaginal delivery. The overall rate of breast-feeding was 41.7%. Forty-five patients (34.1%) were breast fed at the time of diagnosis. Birth weights of 85 patients (64.4%) were within normal range (appropriate for gestational age) and 25 patients (18.9%) had weights lower than the 10th percentile (small for gestational age [SGA]). Twenty-three patients (17.4%) weighed less than the 10th percentile at diagnosis. No patients received blood transfusions or blood products.
2. Chief complaints of patients with CMV infection
Fever was the most common complaint of patients (39.4%, 52 cases) through all ages. Jaundice (20.4%, 27 cases) was the most common presentation among patients younger than 3 months. Other presentations were high aminotransferase (14.4%, 19 cases), growth retardation (6.8%, 9 cases), cough (5.3%, 7cases), 4 cases had vomiting, and diarrhea was present in most of the cases. Additionally, there were symptoms of hepatomegaly, poor oral intake, dyspnea, and seizures.
3. Clinical characteristics of CMV infection
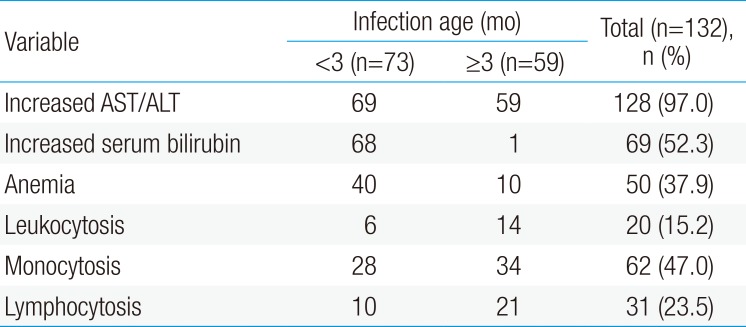
Most infants who presented with jaundice were less than 3 months old (93.2%). Upper respiratory tract symptoms included pneumonia in 48 patients (36.4%), gastrointestinal symptoms, including vomiting in 34 patients (25.8%), intra-uterine growth restriction in 27 patients (20.5%), and failure to thrive in 23 patients (17.4%). Hepatosplenomegaly was noted in 2 patients (1.5%). Other symptoms including lymphadenopathy, skin symptoms, and seizures are presented in Table 1.
An infant who was born at full-term through vaginal delivery weighing 3.3 kg presented with prolonged jaundice at 57 days of life. The levels of aspartate aminotransferase and alanine aminotransferase were 88 and 33 IU/L, respectively. Total and direct bilirubin levels were 8.38 and 6.4 mg/dL, respectively, and serial CMV PCR on blood was positive. The patient underwent comprehensive examination including Tc-99m mebrofenin hepatobiliary scintigraphy for sustained presence of acholic stool and icteric skin color. These studies showed tracer retention in the liver and no uptake in the bowel, confirming biliary atresia. Hence, the patient under went Kasai operation. Only one patient showed microcephaly at diagnosis; however, brain ultrasonography was unremarkable on 40 patients including the infant with microcephaly. There were no cases with hearing loss.
4. Biochemical characteristics of CMV infection
Hepatic abnormalities in the form of cholestasis, identified by elevated total and direct bilirubin levels in serum, were observed in 69 patients (52.3%). Hepatocellular injury, identified by elevated serum alkaline phosphatase and/or gamma glutamyl-transferase levels, were observed in 128 patients (97.0%).
Peak serum levels (range, [median]) of the following markers were, total bilirubin, 0.11 to 21.97 mg/dL (median, 1.93 mg/dL); direct bilirubin, 0.03 to 7.38 mg/dL (median, 1.11 mg/dL); aspartate aminotransferase, 22 to 1,838 IU/L (median, 219 IU/L); and alanine aminotransferase, 5 to 1,517 IU/L (median, 251 IU/L). The duration of aminotransferase elevation ranged from 2 to 48 weeks.
The hematologic findings of hepatic CMV infection revealed anemia, leukocytosis, and monocytosis. Anemia (serum hemoglobin <11×106/µL) was noted in 50 patients (37.9%); leukocytosis (serum white blood cell >18,000/µL) in 20 patients (15.2%); and monocytosis in 62 patients (47.0%). Lymphocytosis had in 31 patients (23.5%). No patients had coagulopathy, or thrombocytopenia.
The mean value of serum total bilirubin was 3.42 mg/dL in patients younger than 3 months old (n=73), and the mean value of serum total bilirubin was 0.35 mg/dL in patients older than 3 months old (n=59). Hepatic CMV infection may appear as hyperbilirubinemia in children younger than 3 months (P<0.001). Other hematologic findings were not different between the 2 groups (Table 2).
5. Immunological markers of CMV infection
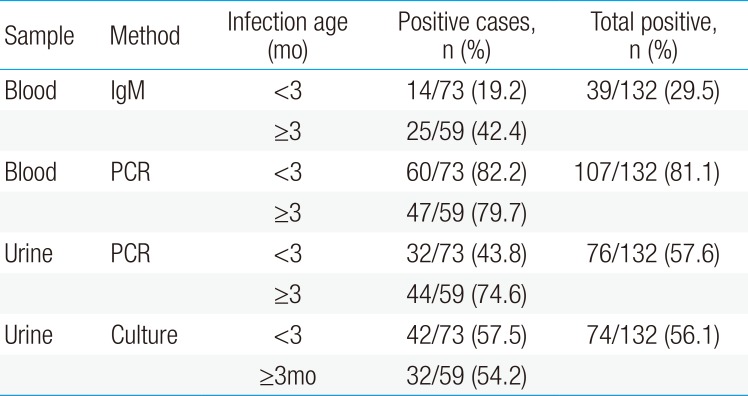
In this study, we enrolled patients who were positive for CMV PCR in serum, anti-CMV IgM, and/or urine PCR or CMV virus culture. The blood CMV PCR was elevated in 107 patients (81.1%), and serum anti-CMV IgM was positive in 39 patients (29.5%). Urine CMV PCR and culture were positive in 76 (57.6%) and 74 patients (56.1%), respectively (Table 3).
6. Clinical course of hepatic CMV infection
The initial acute clinical symptoms such as fever, cough, vomiting, and diarrhea had completely resolved immediately. Eighty-three out of 128 patients with abnormal aminotransferase levels were followed-up after diagnosis. Most patients were followed up at 2-week intervals. Thirty-seven out of 83 patients showed normal levels of liver enzymes, while 38 patients showed progressively improving status at 2 months. In addition, 8 out of 83 patients showed fluctuations in liver enzyme levels, but the patients were in good general condition.
After 2 months, 46 patients were followed up at intervals of 4 weeks. Thirty-three out of 46 patients showed improvements in liver enzyme levels and recovered to normal levels at 4 months. Eight patients recovered to normal aminotransferase levels within 5–6 months. The remaining five patients showed fluctuations in liver enzymes, but they recovered within a year. Eventually, all patients with follow-ups improved their aminotransferase levels without ganciclovir. Therefore, the clinical outcome of CMV hepatitis in these children was considered favorable.
Discussion
CMV infection is one of the main causes of infantile hepatitis and cholestasis. Infantile CMV infection may occur due to either intrauterine or perinatal exposure to CMV infected cervical shedding at the time of vaginal birth, or through breastfeeding1011). Congenital CMV infection, which is acquired intrauterine, can occasionally cause severe disease in infants; however, 90% of congenitally infected infants are evidently normal at delivery121314). The diagnosis of congenital CMV infection requires identification of the virus acquired before age 3 weeks because perinatally acquired infections may also begin to manifest at this time. Hence, a positive viral examination obtained in infants older than 3 weeks may simply represent perinatal or breast milk acquisition of the infection and may not be interpreted as evidence of congenital CMV infection12,15,16).
In this study, nine patients with active CMV infection developed liver dysfunction at 3 weeks after birth. They were all born at full term. Five patients were born with normal birth weights, but 4 patients were SGA at birth. Their perinatal histories were unremarkable without neurological symptoms or hearing loss. However, it is possible that these nine infants had congenital infections, as hepatitis is one of the symptoms of congenital CMV infection.
In this study, fever was the most common symptom at presentation. In addition, jaundice was the most common clinical feature of hepatic CMV infection in patients younger than 3 months old. Regardless of age, increased aminotransferase activities in serum (97%) and hyperbilirubinemia or cholestasis (52.3%) were commonly observed in our patients. This was consistent with results obtained by Liberek et al.17), which showed that increased aminotransferase activities in serum (83%), as well as hyperbilirubinemia and cholestasis (over 40%), were commonly observed. Our data showed that elevated serum alkaline phosphatase and gammaglutamyl-transferase were common findings of hepatic abnormalities, while hypoglycemia and hyperammonemia were uncommon.
Previous studies have reported that transaminases reached the highest levels (<200 U) during the second or third week of infection, decreasing to normal values by the fifth week18). In our study, transaminases increased up to 1,838 IU/L, and most patients showed a significant decrease in transaminases to normal values at 4 months in the follow-up group. The peak total bilirubin levels in serum ranged from 0.11 to 21.97 mg/dL. The median duration of hyperbilirubinemia was 8 weeks and that of alanine aminotransferase elevation was 14 weeks.
Although there are several speculations that CMV causes biliary atresia, so far there is no solid evidence indicating the virus as a causative agent192021). In our series, only one infant aged 57 days presented with prolonged jaundice, showing positive results on serial CMV blood PCR and confirmed biliary atresia.
The laboratory tests used for serological diagnosis of CMV infection are anti-CMV IgM, CMV PCR, urine virus cultures, and urine CMV PCR. In this study, the serum CMV PCR was positive in most patients (81.1%, 107 cases), while anti-CMV IgM was positive in only a few patients (29.5%, 39 cases). The anti-CMV IgM negative patients were mostly under 3 months old. Because young infants have immature immune systems, which may fail to produce antibodies during acute infections, anti-CMV IgM tests can come back negative. As cell-free virus is not present in the blood of healthy individuals6), detection of CMV DNA in the plasma reflects active CMV infection. The urine CMV PCR or urine CMV virus culture was positive for everyone in the serum CMV PCR or anti-CMV IgM positive patients. Although urine CMV PCR or urine CMV virus culture showed a highly positive rate in this study, the infected hosts excrete the virus for considerable durations in their urine, so viral excretion in the urine alone does not necessarily indicate CMV hepatitis.
Treatment of CMV infection with ganciclovir is indicated in certain situations, but the guidelines for treatment, especially in immunocompetent patients, newborns, and infants are not established yet11). In this study, treatment with ganciclovir was determined to be unnecessary as all patients with jaundice and alanine aminotransferase elevation showed early improvement and there was no neurologic or multiorgan involvement. Patients with jaundice were administered ursodeoxycholic acid. Therefore, the clinical outcomes of CMV hepatitis were considered favorable. However, administration of ganciclovir can be considered in CMV hepatitis patients with severe jaundice, coagulopathy, or severe alanine aminotransferase elevation.
Our study has several limitations. First, as this is a retrospective study, we did not have data identifying maternal infection during pregnancy and history of perinatal period. Hence, congenital CMV infection and perinatal CMV infection were not discerned in this study. Second, this study did not include all patients with hepatic CMV infection. Some patients were lost during follow-up. Another limitation is that our results were not validated by histopathological findings of the liver, because we did not acquire liver biopsies from the patients. In the histopathological examination of liver, the presence of cytomegalic cells and inclusion bodies refers to the intense immune activation against viral attack18). Hence, the immune activation status after CMV infection was not confirmed in this study either. Nevertheless, our data do represent the largest cohort of infants and children reported, because the data were systematically collected in a university hospital in Korea over a decade. Future prospective studies with more patients are needed for precise prognosis.
This paper is significant because it describes a study at a single center for a decade on CMV infection in children. Jaundice was the most common clinical feature of hepatic CMV infection in infants younger than 3 months, while fever was present in children of all ages. Real-time PCR assays were useful for investigating the etiology of hepatitis in this study. Suspicious diagnosis of CMV infection is needed when unexplained fever without focus, prolonged jaundice, and abnormal levels of liver enzymes are observed. It is important to detect hepatic CMV infection in infants, as it may progress to portal hypertension and cirrhosis; hence, timely diagnosis can improve the course of illness and reduce complications22). However, in most immunocompetent patients, hepatic CMV infection without the involvement of other organs in children is self-limited without requiring antiviral treatment and showed favorable outcomes in this study.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.