IgE-mediated food allergies in children: prevalence, triggers, and management
Article information
Abstract
Food allergy (FA) is a serious health problem, and severe FA such as food-induced anaphylaxis can often be life threatening. The incidence of FA has been increasing especially in children. They usually develop early in life and affect up to 10% of children. The 2 most common food allergens worldwide are milk and eggs, while the third one varies depending on the countries: peanuts in the United States and Switzerland, wheat in Germany and Japan, tree nuts in Spain, sesame in Israel, and walnuts in Korea. These common food allergens are different and difficult to identify because of differing study methodologies, population, geography, age, and dietary exposure patterns. The current management of FA relies on the strict avoidance of culprit allergens, the prompt treatment of allergic reactions, including epinephrine use for food-induced anaphylaxis, monitoring, and education to prevent further reactions. Newer approaches for tolerance induction to FA and FA immunotherapy have been under investigation but are not yet ready for real-world application. Thus, consistent and systematic education of patients, caregivers, and food-handling people is of primary importance for the management and prevention of FA reactions. This review assesses and compares IgE-mediated FA in children in Korea and other countries, with a focus on summarizing the prevalence, common triggers, and management of FA.
Introduction
Food allergy (FA) is a serious health issue, and food-induced anaphylaxis can often be life threatening. FA can be induced by IgE-mediated, non–IgE-mediated, mixed, or cell-mediated immune reactions to any routes of exposure to culprit foods. However, in most children, FA is mainly caused by IgE reactions following the ingestion of causative food proteins1). Various organs such as the skin, gut, respiratory tract, and cardiovascular system can be affected by FA. Acute severe and near-fatal systemic reactions, so-called anaphylaxis, may occur in various FA and are considered medical emergencies12).
FA usually develops early in life and affects up to 10% of children and 6% of adults and the prevalence of FA has increased worldwide in recent decades12345678). As FA is mainly prevalent in infants and preschool children, the 2 most common food allergens worldwide are milk and eggs, while the third one varies depending on the countries: peanuts in the United States and Switzerland, wheat in Germany and Japan, tree nuts in Spain, sesame in Israel, and walnuts in Korea126789). The main culprit foods are different and difficult to identify because factors such as allergy definition, study population, methodologies, geographic variation, age, dietary exposure, and other factors may influence study results69). The current approach to the management of FA still relies on allergen avoidance and prompt treatment of acute reactions, especially for food-induced anaphylaxis. Successful management of FA requires significant patient, parent, and other caregiver education, including understanding of the disease, confirmation of culprit foods, avoidance, and prehospital management, including epinephrine use in anaphylaxis. Furthermore, a specific legislation is needed in order to oversee food labeling and manufacturing as well as increasing access to epinephrine, availability of advocacy organizations, and access to numerous educational resources developed by FA experts1).
Compared to Western countries and Japan, large-scale all-age studies of FA have not yet been performed in Korean children. This paper offers a detailed review of the prevalence and triggers of IgE-mediated FA in Korean children and summarizes the general concepts of FA management and prevention.
Prevalence of IgE-mediated FA in children
Reports on the prevalence of FA in the general population are generally based on nationwide questionnaire-based studies, although some studies rely on regional cohorts or self-reported assessments. However, these studies lack detailed information regarding the triggers, clinical and laboratory characteristics, and symptom-provoking threshold levels of triggering foods, and thus, their relevance is limited. In contrast, large-scale nationwide case studies performed by food allergists may report more precise and practical information, even though they do not represent the prevalence in the general population.
Several studies in Korea have assessed the FA prevalence in different age groups in the general population310111213). As part of the International Study of Asthma and Allergies in Childhood (ISAAC), a series of nationwide population studies were performed in 1995, 2000, and 2010 to determine the prevalence of allergic diseases in Korean children. Oh et al.10) used data from the 1995 and 2000 surveys to compare the prevalence of FA in elementary and middle school-aged children. In their study, the lifetime prevalence of FA was not significantly different in 1995 and 2000 among 6- to 12-year-old (10.9% in 1995 and 8.9% in 2000) and 12- to 15-year-old children (11.3% in 1995 and 12.6% in 2000). The 12-month prevalence of FA also did not differ significantly in 1995 and 2000 in both age groups (6- to 12-year-olds: 6.5% in 1995 and 5.7% in 2000; 12- to 15-year-olds: 7.4% in 1995 and 8.6% in 2000). On the other hand, the lifetime prevalence of FA ever diagnosed by a medical doctor was 4.7% and 5.1% among 6–12 and 12- to 15-year-olds, respectively, in 2000. As a part of the ISAAC Phase III, Ahn et al.11) reported the findings of another population-based cross-sectional study of FA in more than 30,000 randomly selected participants among the first grade of elementary school (6–7 years old) and middle school children (12–13 years old). In this study, the prevalence among 6–7 and 12- to 13-year-old students was 15.0% and 12.5%, respectively, while the actual rates were 3.3% and 4.5% in each age group, respectively. Furthermore, Park et al.3) performed another questionnaire-based cross-sectional population study in young children between September and October 2011. In this study, the questionnaires were completed by the parents of children aged 0–6 years recruited from 301 public child-care centers in Seoul. A total of 16,749 children were included, and the prevalence of ‘perceived FA, ever,’ ‘immediate-type FA, ever,’ and ‘immediate-type FA, current’ was 15.1%, 7.0%, and 3.7%, respectively. And “immediate-type FA, current” was reported in 182 of 3,738 children (4.9%) aged ≤2 years, 262 of 7,648 children (3.4%) aged 3–4 years, and 177 of 5,363 children (3.3%) aged 5–6 years. To evaluate trends in the prevalence of FA in Korea, Kim et al.13) conducted and compared several surveys of elementary school children in Seoul, Korea in 1995, 2000, 2005, 2008, and 2012. The prevalence rates of ‘FA diagnosis, ever’ were 4.6%, 5.2%, 6.4%, 5.5%, and 6.6% in 1995, 2000, 2005, 2008, and 2012, respectively (P value for trend <0.001). The authors concluded that the prevalence of diagnosed FA in Seoul has been increasing over the last 20 years. Taken together, the prevalence of childhood FA in the general population is lower than that in Western countries but is within the 2%–10% reported general range. On the other hand, the prevalence rates of FA in Korea are similar to those of Western and other Asian countries, while the rankings of common triggers differ between countries1267891415). However, the reported prevalence or incidence of childhood FA should be compared with careful understanding and clear interpretation of the different study methods, subject age, and other factors. The prevalence of FA associated with individual food items and the occurrence of food-induced anaphylaxis will be discussed later in this review.
Main triggers of IgE-mediated FA in children
Over 90% of FA cases in Western countries are triggered by eggs, cow milk, peanuts, tree nuts, fish, shellfish, soy, and wheat. However, the prevalence of individual triggering foods is influenced by age, culture, ethnic background, and dietary patterns. Therefore, the top-ranking causative foods reported differ between studies. In general, the most prevalent food triggers in childhood FA are eggs and cow milk, while the third and fourth most common triggers differ depending on geographical region, age, and regional dietary patterns1459141516). A recent systematic review estimated the common FA prevalence of European studies published from January 1, 2000 to September 30, 201216). In this study, data reported from four electronic databases were pooled using random-effects meta-analyses, and 50 studies were included in a narrative synthesis, while 42 studies were included in the meta-analyses. Although there was a significant heterogeneity between the studies, the overall pooled estimates for all age groups of self-reported lifetime prevalence of FA in the general population to cow milk, eggs, wheat, soy, peanuts, tree nuts, fish, and shellfish were 6.0%, 2.5%, 3.6%, 0.3%, 0.4, 1.3%, 2.2%, and 1.3%, respectively. However, the prevalence of food-challenge-defined allergies to the same triggers was lower than the self-reported lifetime prevalence. The prevalence of allergies to cow milk, eggs, wheat, soy, peanuts, tree nuts, fish, and shellfish was 0.6% (95% confidence interval [CI], 0.5–0.8), 0.2% (95% CI, 0.2–0.3), 0.1% (95% CI, 0.01–0.2), 0.3% (95% CI, 0.1–0.4), 0.2% (95% CI, 0.2–0.3), 0.5% (95% CI, 0.08–0.8), 0.1% (95% CI, 0.02–0.2), and 0.1% (95% CI, 0.06–0.3), respectively. FAs to cow milk and eggs were more common among younger children, while FA to peanuts, tree nuts, fish, and shellfish were more common among older children. In another meta-analysis of 51 publications, the prevalence of self-reported FA for 5 foods (cow milk, eggs, peanuts, fish, and crustacean shellfish) was 12% in children. However, there was a marked heterogeneity between studies regardless of the type of assessment or food item considered which persisted in most analyses even after age stratification. In that study, self-reported FA varied 1.2%–17% for milk, 0.2%–7% for eggs, 0%–2% for peanuts and fish, 0%–10% for shellfish, and 3%–35% for any food6). Besides the common allergenic foods, several foods such as sesame, mustard, buckwheat, and kiwi fruit are important in some countries because of regional dietary patterns151718192021).
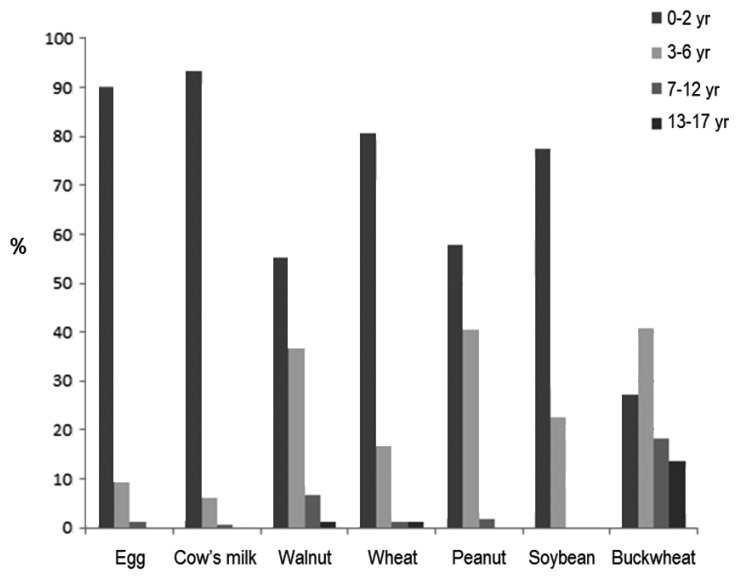
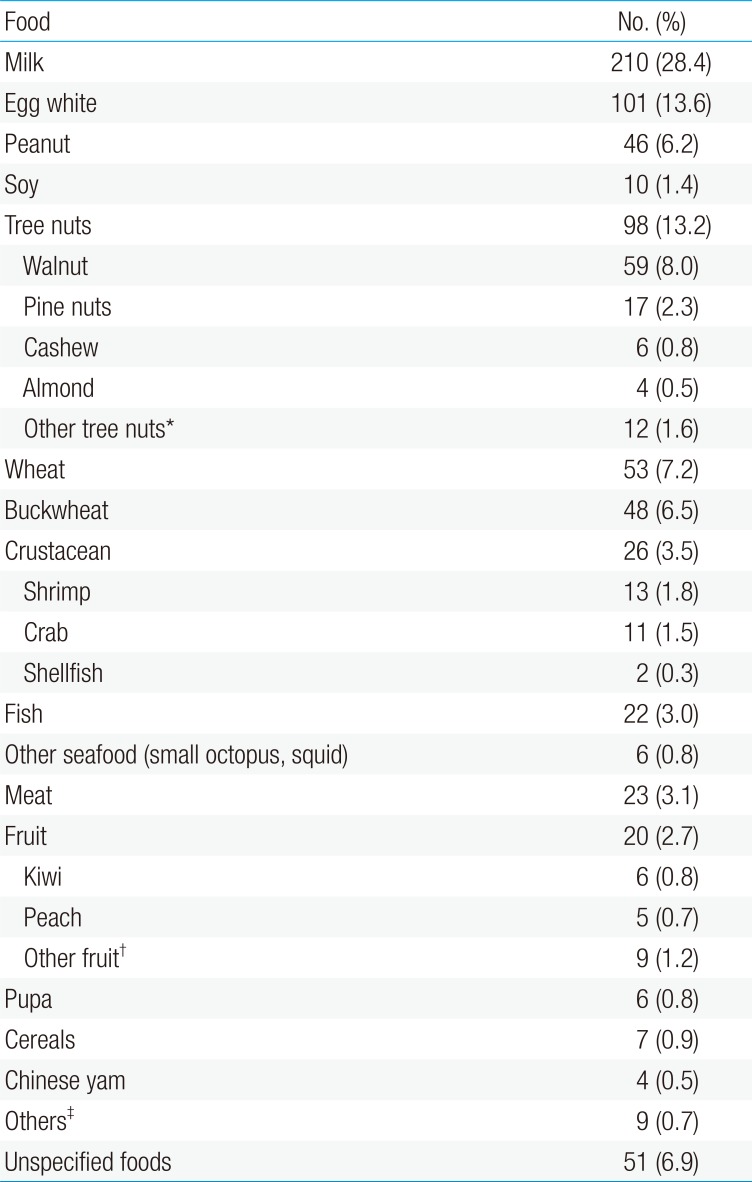
In Korea, several recent large-scale studies have assessed the triggers of childhood FA, including multicenter case studies on food-induced anaphylaxis3512222324). In a Korean population study of children aged 0–6 years in Seoul, chicken eggs (126 of 621, 20.3%) were the most frequent individual food item cause, followed by cow milk (82 of 621, 13.2%) and peanuts (58 of 621, 9.3%). Fruits (114 of 621, 18.4%), tree nuts (90 of 621, 14.5%), and crustaceans (85 of 621, 13.7%) were the most common allergenic food groups3). In a single hospital birth cohort study of 1,177 infants, Kim et al.12) reported a prevalence of immediate-type FA of 5.3%. In their study, the 3 common FA triggers were eggs, cow milk, and peanuts/nuts. As shown in Table 1, the 3 most common triggers of food-induced anaphylaxis were cow milk, egg white, and tree nuts in a recent multicenter study in Korean children and adolescents22). Lee5) recently reported the results of a retrospective multicenter case study of FA in Korea (supported by a 2015 grant from the Korea Ministry of Food and Drug Safety), which was performed by the study group for Food Allergy and Atopic Dermatitis (FAAD) in the Korean Academy of Pediatrics Allergy and Respiratory Diseases. Using high-quality clinical report forms (CRFs) developed by FAAD, this study included valuable data on FA in 2,056 Korean FA children and performed detailed analysis on age-based triggers, clinical symptoms, places and food exposure patterns, laboratory profiles, etc. Data from 2,901 cases of immediate-type FA in 2,056 children aged 0–18 years were collected by means of 7-page detailed CRFs in 14 tertiary hospitals in Korea between September 2014 and August 2015. In that study, FA was diagnosed by pediatric allergists based on a convincing history of reproducible symptoms within 2 hours after exposure to single food items (regardless of test results for sensitization) or an onset of symptoms between 2 and 8 hours only if sensitization was proven by confirmative tests. Among the 2,056 children, 92.5% were less than 7 years of age, and the 12 major triggering foods were chicken eggs (27.4%), cow milk (26.6%), walnuts (7.2%), wheat (6.2%), peanuts (5.5%), soybeans (2.4%), shrimps (2.2%), buckwheat (1.7%), crabs (1.5%), almonds (1.4%), pine nuts (1.3%), kiwi fruits (1.3%), followed by peaches, apples, beef, pork, tomatoes, mackerel, chicken, and cashew nuts5). In this study, an age-based analysis was performed by categorizing the patients into 4 age groups. The 3 most common causative foods were different for each age group. In young children (0–2 years), cow milk and chicken eggs accounted for more than two-thirds of FA cases. In preschool children between 3 and 6 years of age, walnuts (15.0%) were the most common cause of FA, followed by chicken eggs (13.5%) and cow milk (11.5%). In children between 7 and 12 years of age, walnuts and chicken eggs (10.4% each) were the most common causes of FA, followed by cow milk (9.0 %) and shrimps (8.2%). In adolescents between 13 and 18 years of age, buckwheat (14.0%) was the top cause of FA, followed by crustaceans. Compared to previous FA studies in Korea312), the third most common trigger of childhood FA was walnuts instead of peanuts, and age-based differences in the major triggers of FA were observed in this large-scale multicenter study. The age of first symptom onset of individual food triggers differed according to the age group5), as shown in Fig. 1.
In addition to these large-scale studies, several small studies have assessed individual FA and major food allergens, re-evaluated the diagnostic decision point of food-specific IgE antibodies, or presented detailed case studies of the clinical and laboratory characteristics of FA in Korean children172526272829303132333435).
Management and prevention of IgE-mediated food allergies
The current management of FA substantially relies on combinations of 4 categories: (1) strict avoidance of culprit allergens; (2) prompt treatment of allergic reactions, including epinephrine use for food-induced anaphylaxis; (3) monitoring and education for the prevention of further reactions; and (4) induction of tolerance to causative food allergens as an emerging approach11436373839).
1. Avoidance of food allergens and education
The prescription of a targeted allergen elimination diet for the treatment of diagnosed or strongly suspected FA is the most fundamental and important approach for FA. Successful elimination diets should include systematic education about proper food preparation and the risks of occult exposure, especially in patients who have experienced food-induced anaphylaxis. Allergen avoidance diets should be specific and limited to the relevant foods based on a confirmed diagnosis in order to minimize both risks of an allergic reaction and overrestriction. However, debates have recently focused on strict vs. minimal avoidance for the prevention of further reactions compared to helping tolerance induction1143739).
In most FA cases, the primary exposure to a food allergen is through ingestion, although some patients can experience symptoms after skin contact or inhalation of food proteins, as reported in Korean children1517262734). Patients, care providers, and all people responsible for preparing or obtaining foods should be educated on how to read food labels in order to avoid specific food allergens. Proper educational materials related to the most common food allergens and general approaches to avoidance in various settings should be provided11437). Recently, fatal cases of food-induced anaphylaxis due to food served in schools have been reported in several countries, including Korea, which underscores the importance of registering students with FA and practicing proper dietary measures in order to improve the safety of school foods in children with FA1251422).
In Korea, as in Western countries, legislation such as the Food Labeling Law for Main Allergenic Foods, first enacted in 2004 and updated in 2015, is intended to improve consumer safety5373940). In addition, special awareness is required regarding cross-contamination or cross-contact, hidden food ingredients, and cross-reacting proteins. Cross-contamination or cross-contact of causative food allergens is a concern for food preparation at home, at school, in restaurants, and other settings. Examples of cross-contact include poor hand washing, shared grills or pans, poorly cleaned utensils, preparation in poorly cleaned workspaces, etc. Hidden food ingredients in sauces, flavors, and toppings are common unexpected triggers in FA patients, and inhalation exposure to food allergens during cooking may also result in symptoms. When prescribing an elimination diet, clinicians must understand differences in potential risk among cross-reactive foods and make appropriate recommendations, despite the difficulties in optimization. Furthermore, elimination diets must be individually tailored, and clinicians should recognize that proper diets may vary from regular exposure to some proteins to strict avoidance because baked eggs or baked cow milk proteins are tolerated by some patients, and the ingestion of baked eggs and milk allergens can accelerate tolerance induction1273739). The Japanese FA guidelines recommend minimal food elimination based on the correct identification of the causative allergens instead of absolute allergen avoidance39). As in other countries, understanding FA itself and the approaches for management or prevention are poor in Korea, indicating that more concrete and customized FA education is needed for various target groups.
2. Management of the acute symptoms of FA
Several drugs are helpful for the management of acute FA symptoms. Antihistamines are used to manage nonsevere allergic reactions such as simple skin manifestations, but intramuscular injection of epinephrine is the first-line treatment option for acute severe systemic reactions such as anaphylaxis. Even though antihistamines alone are administered for local acute reactions, children should be monitored, and epinephrine should be administered immediately if the symptoms become more severe. For patients experiencing food-induced anaphylaxis, self-injectable (auto-injectable) epinephrine prescriptions are strongly recommended for the proper management of future occurrences of anaphylaxis in children around or over 15 kg in weight. There are minor differences between countries in the relative or absolute indications for prompt use or prescription of epinephrine auto-injectors, and the understanding and use of these potentially life-saving drugs are not satisfactory in patients or primary practitioners. The decision to prescribe an epinephrine auto-injector depends on the risk of food-induced anaphylaxis, and there are suggested indications for the prescription of self-injectable epinephrine in FA. Generally accepted absolute indications include previous cardiovascular respiratory reactions to a food and FA with coexisting persistent asthma, while the relative indications include any reaction to small amounts of a food item (e.g., airborne food allergens or skin contact), history of a previous mild reaction to peanuts or tree nuts, distance from home to medical facilities, and FA reaction in teenagers1441). In addition, buckwheat is considered a potent anaphylactic food in Korea and Japan and might be a risk factor for the development of food-induced anaphylaxis52139). In the Korean market, Jext is commercially available (Korea Orphan Drug Center, http://www.kodc.or.kr) with a doctor prescription. The number of prescriptions has increased annually for the past 5 years22).
3. FA immunotherapy
In addition to the recent recommendations for managing FA, which include a combination of avoidance, education, and acute treatment of symptoms, allergen-specific immunotherapy has been studied as a more active treatment of FA that induces tolerance to culprit allergens. Recently, oral, sublingual, and epicutaneous immunotherapies have been studied for their effectiveness against FA; among these, oral immunotherapy is most commonly investigated114373842).
The goal of food immunotherapy remains controversial, and the evidence of its effectiveness has been limited to the research setting rather than to practical applications37384243). While some researchers feel that the goal should be to induce desensitization and real tolerance induction (sustained unresponsiveness), others feel that a small amount of tolerance that, for instance, allows patients to tolerate an accidental bite of an offending food, is clinically and emotionally significant42). So far, various rates of desensitization (36% to 100%) and tolerance (28% to 75%) have been induced by FA immunotherapy, and no single protocol has been shown to be both effective and safe38). Adverse reactions to food immunotherapy have usually been localized, but severe systemic reactions have also been observed. Although immunotherapy cannot yet be recommended for routine practice, the results of recent studies prove to be promising for the treatment of FA137384243).
4. Prevention of IgE-mediated FA
In order to minimize accidental exposure to culprit foods, for decades, FA guidelines have urged parents to avoid exposing their children to foods such as eggs, peanuts, and fish early in life, based on the idea that early exposure led to allergic sensitization. However, the compelling results of the recent Learning Early About Peanut Allergy trial44) showed that the early consumption of peanuts by high-risk infants dramatically decreased their risk of developing peanut allergies. Based on such evidence, many FA guidelines currently recommend introduction of peanuts to high-risk infants as a primary preventive strategy45). However, another randomized trial on the early introduction of allergenic foods (peanuts and chicken eggs) in breastfed infants did not show the efficacy of early introduction of allergenic foods in an intention-to-treat analysis46). Therefore, additional randomized clinical studies are necessary in order to determine if the prevention of FA by means of early introduction of individual food items is effective and recommendable in general.
Conclusions
Accumulating evidence suggests that the prevalence of FA has been increasing in recent decades, and its prevalence and main triggers vary according to patient age and race, geographical area, and regional dietary patterns. This review provided a detailed description of the prevalence and main triggering foods of FA in the general population and in multicenter hospital-based case studies in Korea. These findings may help physicians diagnose, manage, and educate children with FA. The current management of FA relies on the combinations of strict avoidance of culprit allergens, prompt treatment of allergic reaction, including epinephrine use for food-induced anaphylaxis, monitoring, and education to prevent further reactions. An emerging approach for tolerance induction to culprit food allergens has been under investigation, but it is not yet ready for application in the clinical practice. Proper and systematic education for patients and all type of caregivers is the most important tool for the management and prevention of further FA reactions.
Acknowledgments
This work was partly supported by a grant (MFDS) from Korea Ministry of Food and Drug Safety in 2015. Nation-wide multicenter case studies of food allergy and anaphylaxis were conducted by the study group for Food Allergy and Atopic Dermatitis (FAAD) in the Korean Academy of Pediatric Allergy and Respiratory Diseases.
Notes
Conflict of interest: No potential conflict of interest to this article was reported.