Case of seropositive allergic bronchopulmonary aspergillosis in a 10-year-old girl without previously documented asthma
Article information
Abstract
Allergic bronchopulmonary aspergillosis (ABPA) is a hypersensitivity lung disease due to bronchial colonization of Aspergillus fumigatus that occurs in susceptible patients with asthma or cystic fibrosis. A 10-year-old girl was referred to the Department of Pediatric Pulmonology for persistent consolidations on chest radiography. Pulmonary consolidations were observed in the right upper and left lower lobes and were not resolved with a 4-week prescription of broad-spectrum antibiotics. The patient had a history of atopic dermatitis and allergic rhinitis but no history of asthma. She had no fever but produced thick and greenish sputum. Her breathing sounds were clear. On laboratory testing, her total blood eosinophil count was 1,412/mm3 and total serum IgE level was 2,200 kU/L. Aspergillus was isolated in the sputum culture. The A. fumigatus-specific IgE level was 15.4 kU/L, and the Aspergillus antibody test was also positive. A chest computed tomography scan demonstrated bronchial wall thickening and consolidation without bronchiectasis. An antifungal agent was added but resulted in no improvement of pulmonary consolidations after 3 weeks. Pulmonary function test was normal. Methacholine provocation test was performed, revealing bronchial hyperreactivity (PC20=5.31 mg/mL). Although the patient had no history of asthma or bronchiectasis, ABPA-seropositivity was suspected. Oral prednisolone (1 mg/kg/day) combined with antifungal therapy was started. Pulmonary consolidations began decreasing after 1 week of treatment and completely resolved after 1 month. This is the first observed and treated case of seropositive ABPA in Korean children without previously documented asthma.
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is a hypersensitivity lung disease due to bronchial colonization by Aspergillus fumigatus that occurs in susceptible patients with asthma or cystic fibrosis, with a prevalence of 2%-15% in western countries12). However, only one case of ABPA with central bronchiectasis was reported in Korean children3). The clinical presentation of ABPA can range from mild bronchospasm to fibrotic parenchymal disease4). ABPA diagnosis might be easily missed or delayed because its clinical presentation is often indistinguishable from those of more common pulmonary disorders5). Here, we report an unusual case of seropositive ABPA in a 10-year-old Korean girl without previous history of asthma.
Case report
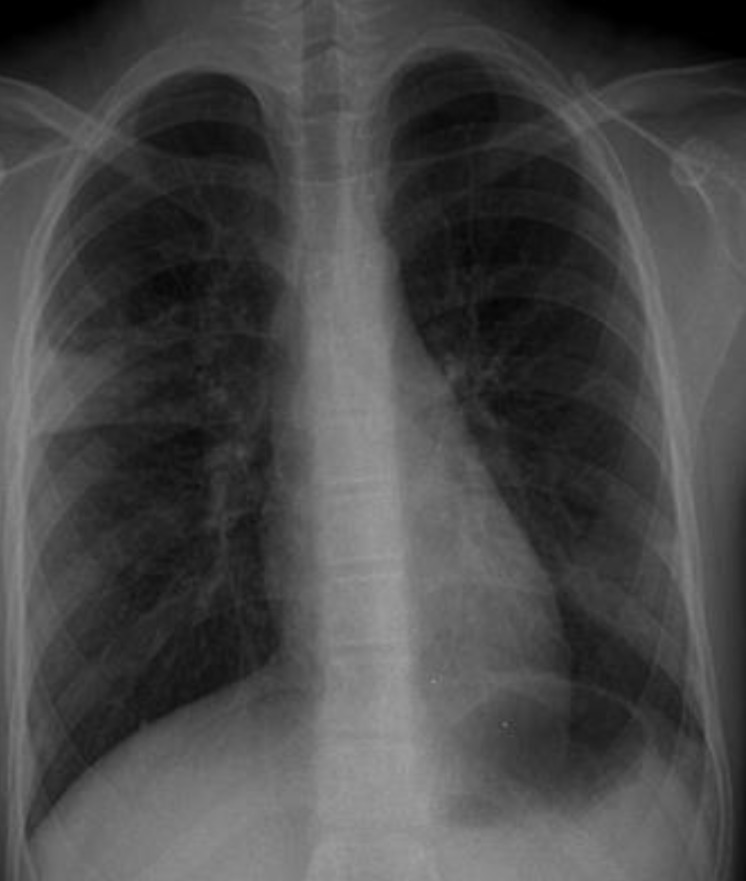
A 10-year-old girl presented with a 4-month history of intractable cough and purulent rhinorrhea without fever. A review of systems was unremarkable. Medical history revealed that the patient had atopic dermatitis, allergic rhinitis, and 2 times of pneumonia. But, she had never been diagnosed with asthma, although she had a history of chronic cough since childhood. Chest breathing sounds were clear without rales or wheezing. Radiologic findings showed segmental consolidations in the right upper and left lower lobes (Fig. 1). There was no sign of pneumothorax or pleural effusion. The patient had been treated with broad spectrum antibiotics (amoxicillin-clavulanic acid and clarithromycin) for 4 weeks but chest x-ray failed to show improvement. She was referred to the Department of Pediatric Pulmonology.
Complete blood cell counts were as follows: white blood cells, 13,500/mm3 (neutrophils, 59.2%; lymphocytes, 24.6%; monocytes, 2.7%; and eosinophils, 13.1%); hemoglobin, 13.5 g/dL; and platelets, 450,000/mm3. The erythrocyte sedimentation rate was 43 mm/hr. C-reactive protein was less than 0.5 mg/dL. A chest computed tomography (CT) scan demonstrated bronchial wall thickening and consolidation without bronchiectasis in the right upper and left lower lobes (Fig. 2).

Computed tomography scan of chest shows low density lesion and bronchial wall thickening without central bronchiectasis in right upper (A) and left lower lobes (B).
Etiological evaluation for pneumonia did not show causative pathogens. No respiratory viruses were detected in the polymerase chain reaction (PCR) from nasopharyngeal aspirates. Blood and sputum cultures for bacteria were negative. Antibodies for Mycoplasma pneumoniae and urine Streptococcus pneumoniae antigen test were negative. Parasitic infestations were excluded by the negative results of tests for specific antibodies for Cysticercus, Sparganum, Paragonimus, and Clonorchis. Tests for pulmonary tuberculosis including Mantoux-test, acid-fast bacilli staining and culture from the sputum were all negative. Stool examination was normal. In one of the three repeat sputum cultures, Aspergillus species was isolated. Antifungal agents (100 mg of voriconazole twice per day) were added to broad spectrum antibiotics. However, pulmonary consolidation did not improve until 3 weeks after adding antifungal agent.
Serum total IgE level was 2,200 kU/L, and specific IgE levels for Dermatophagoides farinae, Dermatophagoides pternonyssinus, and Alternaria were 4.15 kU/L, 1.84 kU/L, and 25.5 kU/L, respectively. Serum IgE and IgG antibodies specific to A. fumigatus were positive (>200 U/mL), and antigen was negative. A. fumigatus-specific IgE level was 15.4 kU/L. Serum humoral antibody levels were normal (IgG, 1,163.0 mg/dL; IgM, 168.7 mg/dL; and IgA, 136.8 mg/dL). T-cell subsets were also within normal limits (lymphocytes, 3,612/mm3; CD3+ T cells, 2,636/mm3; CD4+ T cells, 1,950/mm3; CD8+ T cells, 1,300/mm3; and CD4+/CD8+ T cells, 1.5) and the serum levels of C3 and C4 were 146.93 mg/dL and 23.78 mg/dL, respectively. The alpha1-antitrypsin level was also within normal limits (186.3 mg/dL). Pulmonary function test was normal (forced vital capacity [FVC], 2.62 L (81% pred); forced expiratory volume in one second (FEV1), 2.47 L (84% pred); FEV1/FVC, 86%). However, methacholine provocation test showed airway hyperreactivity (AHR) (PC20=5.31 mg/mL). The diagnosis of seropositive ABPA was assumed, and oral prednisolone (1 mg/kg/day) was added to antifungal treatment. After 1 week of oral prednisolone, chest x-ray consolidation began decreasing, and IgE level lowered to 616 kU/L with blood eosinophils of 400/mm3. The patient was treated with oral prednisolone (1 mg/kg/day) for 10 days and tapered down over the following days. One month later, chest x-ray abnormalities were cleared up completely and symptoms improved continuously for the following days (Fig. 3).
Discussion
The pathogenesis of ABPA is not completely understood. Many immune responses appear to be involved, including Aspergillus-specific IgE-mediated type I hypersensitivity reactions, specific IgG-mediated type III hypersensitivity reactions, and abnormal T-lymphocyte responses6).
Due to the absence of preexisting asthma, this case was initially mistaken for atypical pneumonia other than ABPA. Diagnosis is based on a combination of clinical, laboratory, and radiographic finding, and a set of specific diagnostic criteria is established for patients with asthma. Greenberger7) standardized the criteria for diagnosing ABPA in asthma: (1) asthma, (2) proximal bronchiectasis, (3) immediate cutaneous reactivity to Aspergillus species or A. fumigatus, (4) total serum IgE elevation (>417 kU/L or 1,000 ng/mL), and (5) elevated serum IgE-A. fumigatus and/or serum IgG-A. fumigatus. Most patients with ABPA do not fulfill all the criteria, especially early in the course of the disease7).
Asthma is normally considered essential for diagnosis of ABPA. However, there are published cases in which ABPA has occurred without asthma. A systematic MEDLINE search of the literature using the term "allergic bronchopulmonary aspergillosis" revealed 17 articles that have reported ABPA without asthma. In total, there were 36 reported occurrence of ABPA without asthma cases; 2 cases demonstrated bronchodilator reversibility and 1 case revealed AHR to methacholine challenge8). Thus, it is possible that some of these patients will develop asthma later during their disease course. The current patient also did not have pre-existing asthma, but methacholine provocation test showed AHR.
High resolution CT (HRCT) of the chest is the ideal choice for radiological investigation in ABPA. HRCT findings include central bronchiectasis and mucus plugging with bronchocele formation9). This patient did not show central bronchiectasis or mucus plugging on chest CT. In the absence of typical proximal bronchiectasis, the condition is designated seropositive ABPA (ABPA-S) as opposed to ABPA with central bronchiectasis (ABPA-CB)10). According to a recent study in India, almost 25% of ABPA cases are diagnosed as ABPA-S11). Patients with ABPA-S represent the earliest stage of the disease with less severe immunologic findings than ABPA-CB12). To our knowledge, this is the first case of seropositive ABPA without history of asthma and central bronchiectasis on chest CT in Korean children.
Patterson et al.13) have classified ABPA into 5 stages based on asthma severity, and radiographic or laboratory findings. However, it doesn't necessarily mean sequential progression from one stage to the other. Stage I is the initial acute stage of ABPA with many of the typical features of the disease. In stage II, the disease goes into remission; the infiltrates clear, symptoms are reduced, and the serum IgE value falls but usually remains elevated. Stage III is an exacerbation associated with the recurrence of the initial symptoms and a twofold increase in serum IgE levels. Stage IV is the corticosteroid-dependent stage. Patients need continuous corticosteroids either to control their asthma or to prevent a recurrence of ABPA. Stage V is the fibrotic stage. Bronchiectasis and fibrosis develop, which leads to irreversible lung change13). Patients with ABPA-S may be classified as Patterson stages I to IV14). Our case can be categorized as stage I.
Differentiating ABPA from more common pulmonary disorders remains a challenge due to similarities in symptoms5). Because both pneumonia and ABPA may cause pulmonary infiltrations on chest radiographs, it can be problematic to distinguish these two entities7). If the condition fails to improve after a course of antibiotics, clinicians can narrow the differential diagnosis in patients with respiratory complaints and pulmonary infiltrations. Another differentiation point is corticosteroid-responsiveness, which is therapeutically important5). Chest x-ray findings should improve dramatically after corticosteroid therapy, as seen in this case. ABPA treatment is primarily aimed at attenuating immunological activity, usually with corticosteroids12). Greenberger recommended low dose of glucocorticoids; prednisolone 0.5 mg/kg/day for 1 to 2 weeks, then on alternate days for 6 to 8 weeks, followed by tapering by 5 mg to 10 mg every 2 weeks until discontinued7). The duration and dose of therapy should be individualized according the patient's clinical condition. Antifungal agents may have a role in ABPA to reduce the burden of fungal colonization in the lung, thus attenuating the intense inflammatory response and preventing subsequent lung damage2). The antifugal agent voriconazole recently showed a favorable response in some ABPA cases15). However, this patient did not show radiological improvement to therapy with voriconazole for 3 weeks.
Even if a patient has no definite history of asthma or central bronchiectasis on chest CT, clinicians should be alert for potential seropositive ABPA when radiologic consolidations do not improve with appropriate antibiotics, and serum total IgE and blood eosinophil levels are high. Early and correct ABPA diagnosis can reduce unnecessary antibiotic treatments and prevent progression of lung destruction. If diagnosed early and treated promptly, ABPA has a good prognosis. ABPA must be suspected in patients with allergic diseases and unresolved pneumonia regardless of history of asthma.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.