Antibiotics resistance of Helicobacter pylori and treatment modalities in children with H. pylori infection
Article information
Abstract
Pediatric infection with Helicobacter pylori may occur early in childhood and persist lifelong. Global pediatric clinical studies have reported a decreasing tendency in the overall rate of H. pylori eradication. In pediatric patients with H. pylori infection, pediatric patients with peptic ulcer, and the first-degree relatives of patients with a history of gastric cancer, it is commonly recommended that H. pylori strains be eradicated. Antibiotic drug resistance to H. pylori, which has been reported to vary widely between geographic regions, is mainly associated with treatment failure in these patients. It is therefore imperative that the antibiotic resistance rates of H. pylori in children and adolescents be meticulously monitored across countries and throughout geographic regions. This paper particularly focuses on the antibiotic drug resistance of H. pylori and the thearpy of pediatric H. pylori infection cases.
Introduction
Helicobacter pylori infection in young children and adolescents1,2) is a chronic, persistent infection that may lead to chronic gastritis, atrophic gastritis, intestinal metaplasia, and even gastric adenocarcinoma3). As such, it is likely that eradication of H. pylori lowers the risk of developing gastric cancer4,5,6). International guidelines for the treatment of H. pylori infection recommend the use of a 7-day, triple-medication therapy consisting of clarithromycin, either metronidazole or amoxicillin, and either a proton pump inhibitor (PPI) or bismuth citrate as the first-line of treatment7,8,9,10). Nevertheless, antibiotic resistance remains problematic in the treatment of H. pylori infection11,12), requiring careful monitoring of antibiotic resistance to H. pylori in children and adolescents throughout regions and countries13).
This paper particularly focuses on the antimicrobial resistance of H. pylori and the treatment of pediatric H. pylori infection cases.
Antimicrobial resistance of H. pylori in children
A useful means of choosing the best treatment options and/or managing treatment failure14) is obtaining a culture of H. pylori from the gastric mucosa, which helps in determining the antimicrobial susceptibility. However, as compared with other normal intestinal flora, the fragility of H. pylori at room and very low temperatures poses a therapeutic challenge for clinicians15). Further, data regarding the antimicrobial susceptibility of H. pylori in children as compared with that in adults is scarce, owing to the lesser need to perform endoscopy in children as well as because endoscopy is rarely indicated for the treatment of children.
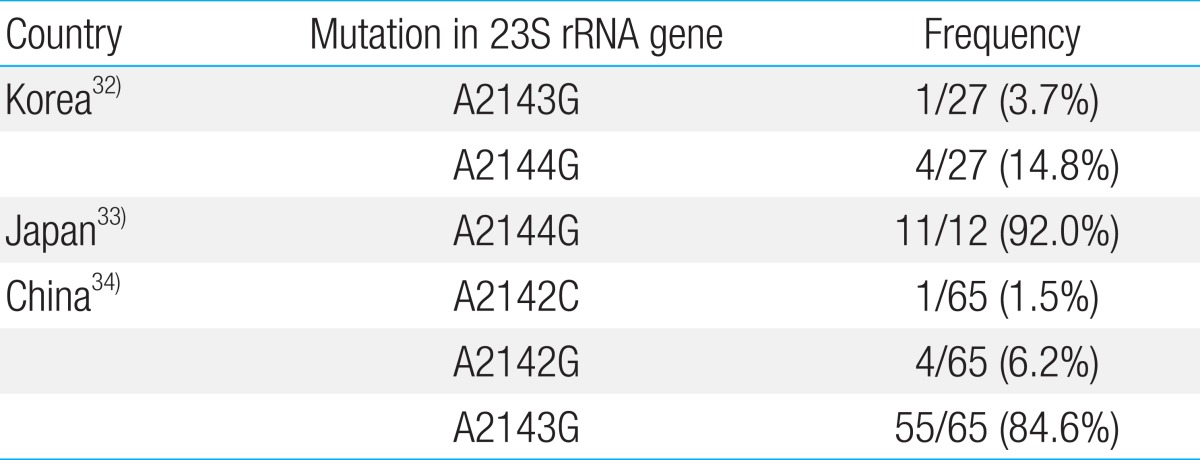
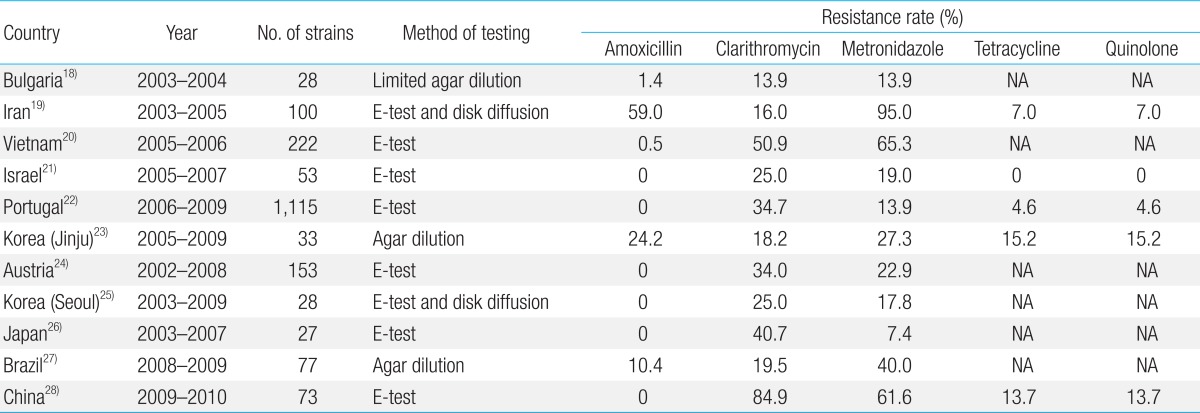
H. pylori resistance rates may vary among patients depending on age, sex, disease state, and regional location. In some countries, antimicrobial resistance has evolved with the use of antibacterial agents3,16). Table 1 shows recent the rates of H. pylori resistance to antimicrobials that are commonly administered to children in 10 countries17,18,19,20,21,22,23,24,25,26,27). The E-test has been established as a reliable modality for measuring antibiotic resistance to all antimicrobials, except for metronidazole for which it overestimates resistance. However, its results should be confirmed using the agar dilution method. Therefore, the agar dilution method has been recommended to test H. pylori resistance28). The serial 2-fold agar dilution method cannot be routinely used to examine the antimicrobial sensitivity because it is expensive and difficult to perform. To date, most of the studies in the antibiotic resistance of H. pylori have been conducted using the E-test method.
Primary antimicrobial resistances of Helicobacter pylori in children according to a review of studies published since 2000
Amoxicillin is a β-lactam antibiotic used to treat numerous bacterial infections (e.g., acute otitis media, streptococcal pharyngitis, pneumonia, impetigo, cystitis, and acute bacterial enteritis). Once administered orally, it is absorbed better and is more effective against H. pylori than other β-lactam antibiotics. According to a meta-analysis of 6 published studies, there have been no reports of amoxicillin resistance (Table 1)20,21,23,24,25,27). In contrast, 2 South Korean studies reported a great discrepancy in the rate of amoxicillin resistance between the 2 main regional areas, with Seoul reported to have a rate of 0% and Jinju, of 24.2%22,24). This discrepancy might be due to differences in the prescription of antibiotics to patients by the medical doctors of these areas. In Jinju, the rate of amoxicillin resistance increased from 19.0% between 1990 and 1994 to 24.2% between 2005 and 200922), and has been estimated at more than 50% in Iran18). These findings suggest that administration of a course of amoxicillin is ineffective for the management of H. pylori infection. In contrast, there have been no reports of amoxicillin resistance in Israel, Portugal, Austria, Japan, China, or, as reported above, in Seoul, South Korea (Table 1), which indicates that amoxicillin administration is a recommended modality for the treatment of H. pylori infection. The causes of this great discrepancy in the rate of amoxicillin resistance among regions and countries remain unclear. This is because amoxicillin is one of the most commonly prescribed antibiotics for children. Further follow-up studies are therefore warranted to evaluate changing trends in the rate of amoxicillin resistance.
Clarithromycin, a macrolide antibiotic that has been commonly prescribed to South Korean children for the treatment of infectious disease since 1997, is the treatment of choice that may be alternatively used with chemotherapy regimens to treat H. pylori infection. A significant correlation has been reported between clarithromycin resistance and the rate of H. pylori eradication in pediatric patients25). The range of clarithromycin resistance that has been reported for 10 countries varies widely, from 13.9% to 84.9% (Table 1), and is higher in 7 countries than the rate previously reported in 14 European countries (<24.0%)29). Moreover, an increasing tendency in the rate of clarithromycin resistance has been observed in Bulgaria17), South Korea22,24), Austria23), and Japan25). Presumably, this increase is due to the increased rate of prescription of clarithromycin for the treatment of respiratory tract infections in children. At the same time, an age-dependent decrease in the prevalence of clarithromycin resistance has been observed in Vietnam, decreasing from 62.7% in children aged 3-6 years to 54% in children aged 7-10 years and to 31.7% in children aged 11-15 years19). The marked increase in the rate of clarithromycin resistance in Vietnam over the past decade may be explained by the extensive administration of clarithromycin for the treatment of respiratory tract infections in children since its inexpensive generic version became commercially available19). It may also be partially explained by the widespread prescription of macrolides for the treatment of children aged 3-10 years.
In patients with H. pylori infection, clarithromycin resistance occurs as a result of mutations of domain V in the 23S ribosomal RNA (rRNA) gene30). Although such mutations have been identified in children in South Korea, China, and Japan (Table 2)27,31,32), the type of mutation varies by country. Whereas the A2144G mutation is the most prevalent mutation in macrolide-resistant strains in South Korea and Japan, the A2143G is the most prevalent in those from China. It has also been reported that erythromycin and azithromycin resistance results from the point mutation of the 23S rRNA gene. However, no studies have reported the rate of cross-resistance among erythromycin, azithromycin, and clarithromycin in the management of H. pylori infection. It is presumed that the low resistance rate is associated with the widespread administration of a course of clarithromycin for the treatment of children with H. pylori infection in such countries as Bulgaria, Iran, Brazil, and South Korea (Jinju).
Currently, metronidazole is widely administered to treat anaerobic bacterial infection, amebic infection, and gynecologic infection. As shown in Table 1, the range of metronidazole resistance in H. pylori isolated from children ranges from 7.4%25) to 95.0%18). This relatively wider range, than that of amoxicillin and clarithromycin, might be due to the differences in the epidemic diseases (e.g., amebic infections) prevalent in different countries. It has been reported that a course of metronidazole might be effective against H. pylori infection in countries such as Iran18), Vietnam19), Brazil26), and China27). In contrast, according to 2 Korean studies, the prevalence of metronidazole resistance has decreased over time in both major regions of South Korea, from 32.8% between 1990 and 1994 to 27.3% between 2005 and 2009 in Jinju22) and from 44.4% between 2003 and 2006 to 5.3% between 2006 and 2009 in Seoul24), which may be attributable to the decreased administration of metronidazole in the treatment of South Korean children.
Several studies regarding the administration of other antimicrobials, such as tetracycline, quinolone, rifabutin, and furazolidone, for the treatment of patients with H. pylori infection have reported tetracycline or quinolone resistance. However, tetracycline is contraindicated for children aged ≤8 years and quinolone for children aged ≤18 years. Review of the findings indicates that children might become vertically infected with tetracycline- or quinolone-resistant H. pylori. The rates of tetracycline and quinolone resistance have both been reported to range from 0% to 15.2%, depending on the regional area (Table 1)18,21,22,27).
Dual antimicrobial resistance is another problem in the management of H. pylori infection. The rate of dual resistance to clarithromycin and metronidazole has been reported as 5.7% in Bulgaria17), 42.0% in Iran18), 28.8% in Vietnam19), 13.0% in Israel20), 4.9% in Portugal21), 15.2% in Jinju, South Korea22), 33.3% in Seoul, South Korea24), 10.9% in Austria23), 7.8% in Brazil26), and 38.4% in China27). A 7-day triple-therapy regimen of a PPI, amoxicillin, and either clarithromycin or metronidazole remains the first-line therapy in regional areas where primary clarithromycin resistance is estimated to range between <15% and 20%14). Use of a clarithromycin-metronidazole regimen should be considered after the antimicrobial sensitivity test in Iran, Vietnam, China, and Seoul, South Korea.
Treatment of H. pylori infections in children
H. pylori infection in pediatric patients is characterized by mild gastritis, normal gross appearance of the stomach, and lower incidence of peptic ulcer than that in adults33,34). In the treatment of these patients, as well as that of pediatric patients with peptic ulcer and that of the first-degree relatives of patients with a history of gastric cancer, eradication of H. pylori strains is commonly recommended13). H. pylori infection should also be suspected in children with iron-deficiency anemia who are refractory to iron therapy, and treatment should be provided as appropriate35).
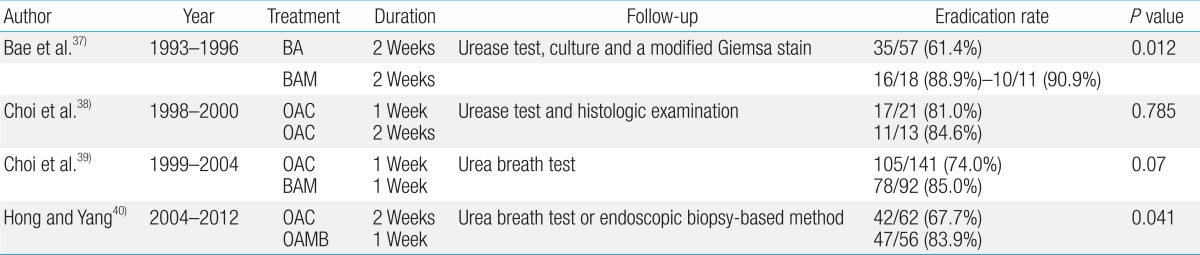
Four studies comparing the effectiveness of different treatment modalities for H. pylori infection in South Korean children all found that a bismuth-based triple therapy was more effective against H. pylori as the first-line of treatment than a PPI-based therapy (Table 3)36,37,38,39). Other studies in South Korea have reported a decreasing tendency in the rate of H. pylori eradication by the administration of omeprazole with amoxicillin and clarithromycin, specifically a decrease from 81.0% between 1998 and 200037), to 74.0% between 1999 and 200038), and to 67.7% between 2004 and 201239). Moreover, it has been reported that the quadruple therapy showed a higher rate of H. pylori eradication than the triple therapy39). Presumably, these results might be due to the inhibitory effects of bismuth on H. pylori growth and the low rate of resistance to amoxicillin or metronidazole in patients in Seoul24).
Correlation between the rate of Helicobacter pylori eradication and the optimal treatment modalities in Korean children
Little is known regarding the pharmacokinetics of antimicrobial agents in children. In a study of 238 H. pylori-infected children comparing the rate of H. pylori eradication using clarithromycin- and metronidazole-based triple therapies between a once daily (q.d.) regimen group, which consisted of patients weighing 13-22 kg, and a twice daily (b.i.d.) regimen group, which consisted of patients weighing 23-45 kg, the rate of H. pylori eradication was found to be lower in the q.d. group than in the b.i.d. group (45.7% vs. 70.9%, respectively)40). This finding suggests that weight-based dosages might be based on either suboptimal dosing recommendations or, because some medications are not available as pediatric formulations, suboptimal dosing itself, unless the medication is adjusted for administration to children14). One study of 62 children, aged <18 years weighing <15 kg, with H. pylori infection who were refractory to metronidazole and clarithromycin found the rate of H. pylori eradication to be 66%-73% after a 2-week course of high-dose amoxicillin, metronidazole, and esomeprazole therapy41). In these children, the most common adverse events were nausea, diarrhea, and vomiting41). Further studies are therefore warranted to determine the appropriate doses of antimicrobials and PPI or bismuth in children weighing <30 kg. Host factors, such as bacterial burden and cytochrome P450 genotype, are mainly associated with treatment outcomes. In addition, the proportion of H. pylori is another factor that is associated with the treatment efficacy as a proportion of the bacteria become attached to the gastric mucosa, where they produce a biofilm28). The H. pylori present are then protected by the thick mucosal layer in the acidic environment, likely greatly reducing the efficacy of many antimicrobials in the acidic pH environment. In light of these findings, antisecretory agents, such as PPIs, have been used to increase the bioavailability of amoxicillin and metronidazole in the treatment of H. pylori infection28). Based on the findings of dose-related studies, it may also be beneficial to prescribe doses of medications higher than those currently recommended for children, such as the doses prescribed for a 14-day course for adults, for children weighing >30 kg40,41).
Recent studies have reported on sequential therapy and concomitant use of probiotics14). In this regimen, a 10-day course comprises a 5-day course of PPI and amoxicillin and a 5-day course of clarithromycin and metronidazole42). Probiotics are used to resolve the treatment-emergent adverse effects and to increase the efficacy of H. pylori eradication14,43). Further studies are therefore warranted to assess the efficacy of sequential therapy and concomitant use of probiotics in children.
Conclusions
Great variability exists in the antimicrobial resistance of H. pylori in children by regional location. Clinicians should therefore consider performance of continuous and serial antimicrobial sensitivity testing and the eradication rate when determining the optimal treatment regimen (e.g., a PPI or bismuth-based regimen), use of antimicrobial agents, and the timing, dosage, and duration of the agents.
Notes
No potential conflict of interest relevant to this article was reported.