Pulmonary hypertension in infants with bronchopulmonary dysplasia
Article information
Abstract
An increase in the number of preterm infants and a decrease in the gestational age at birth have resulted in an increase in the number of patients with significant bronchopulmonary dysplasia (BPD) and secondary pulmonary hypertension (PH). PH contributes significantly to the high morbidity and mortality in the BPD patients. Therefore, regular monitoring for PH by using echocardiography and B-type natriuretic peptide (BNP) or N-terminal-proBNP must be conducted in the BPD patients with greater than moderate degree to prevent PH and to ensure early treatment if PH is present. In the BPD patients with significant PH, multi-modality treatment, including treatment for correcting an underlying disease, oxygen supply, use of diverse selective pulmonary vasodilators (inhaled nitric oxide, inhaled prostacyclins, sildenafil, and endothelin-receptor antagonist) and other methods, is mandatory.
Introduction
Bronchopulmonary dysplasia (BPD) was first described by Northway et al. (1967) as a chronic lung disease of preterm infants who required oxygen supply and mechanical ventilation for acute respiratory distress1). The introduction of prenatal steroid therapy, gentle ventilation techniques, exogenous surfactant treatment, and intensive treatment of patent ductus arteriosus have minimized severe lung injury and improved the survival rate in preterm infants over the 40 years since the introduction of the BPD concept. However, some infants with very low birth weight still develop BPD2). The overall incidence of BPD, which is characterized by the need for oxygen at 36 weeks of gestation, is about 30% for infants with birth weight less than 1,000 g in the United States3) and 9.7% for infants with birth weight less than 1,500 g analyzed in a Korean multicenter study4).
Preterm infants with BPD usually have cardiovascular sequelae, such as pulmonary hypertension (PH), cor pulmonale with right ventricular (RV) hypertrophy, systemic hypertension, left ventricular hypertrophy, and development of systemic-to-pulmonary collateral vessels5). Among these cardiovascular sequelae, PH contributes significantly to the high morbidity and mortality in the BPD patients. Although little is known about the prevalence and mortality rate of PH, the prevalence of PH ranges from 8 to 25% and the mortality rate ranges from 14 to 50% in the BPD patients with PH6-9).
This review focuses on the precise evaluation of the severity of PH and the recent progress in the treatment of PH in the BPD patients.
Development of PH in the BPD patients
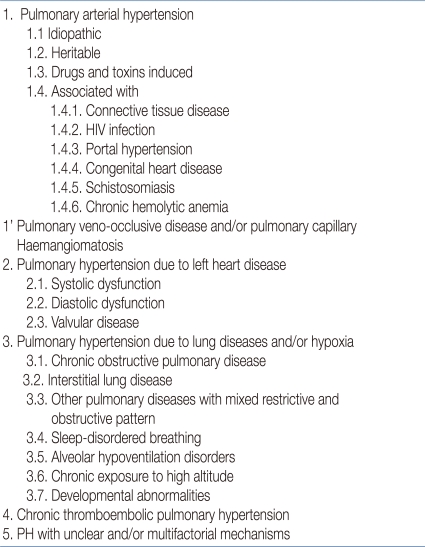
According to the classification of PH by the symposium at Dana Point in 2008, BPD is considered to be a class 3 disorder, which is PH with lung disease and/or hypoxia10) (Table 1). Although the exact pathophysiological characteristics of PH in the BPD patients is unknown, several mechanisms have been proposed. Abnormal lung circulation in the BPD patients, including both anatomical and physiologic abnormality, is related to the development of PH. The patients with BPD show reduction of small pulmonary arteries and an altered distribution of pulmonary arteries within the pulmonary interstitium11), which cause reduction of alveolar-capillary surface area. This reduction in surface area causes impairment of gas exchange and increases the requirement for prolonged oxygen and ventilator treatment, which in turn, raise the risk for severe PH. These pulmonary vascular changes, which are characterized by decreased vascular growth and structure, in preterm infants also contribute to the abnormal cardiopulmonary sequelae of BPD12). Physiologic abnormalities of lung circulation include increased vascular tone and reactivity, hypertensive remodeling, and decreased vascular growth12, 13). Previous studies using cardiac catheterization have shown that even mild hypoxia causes marked elevation of pulmonary artery pressure in the patients, including preterm infants with BPD14). To achieve normal gas exchange, the lung tissue must undergo continuous growth and maintenance of the complex system of airways and vessels. However, disruption of angiogenesis and inhibition of pulmonary vascular growth during a critical period of postnatal lung growth impairs pulmonary alveolarization, which contributes to BPD and increases the risk for PH15).
Systemic-to-pulmonary collateral vessels, including prominent bronchial arteries, also contribute to PH development in the BPD patients. Significantly large collateral vessels may result in significant shunt flow to the lung and cause pulmonary congestion, ultimately requiring more ventilatory support and medications16). In some cases, patients showed improvement of PH after embolization of large collateral vessels and required less supplemental oxygen and ventilatory support12).
Diagnosis of PH in the BPD patients
Early detection of PH may lead to earlier treatment with more aggressive respiratory support and cardiac medications, including pulmonary vasodilators, and other procedures to improve the outcome in the BPD patients. However, the diagnosis of PH in BPD patients is difficult because manifested symptoms and signs of PH may be subtle and may sometimes overlap with those of respiratory problems or infection. In this respect, screening of PH in the BPD patients is necessary to decrease morbidity and mortality from PH.
PH in the BPD patients is defined as a condition characterized by a mean pulmonary artery pressure greater than 25 mmHg and pulmonary capillary wedge pressure less than 15 mmHg on cardiac catheterization in the resting state10). Cardiac catheterization is the gold standard for the diagnosis of PH; however, since this procedure is very invasive and is not performed in all the centers with neonatal intensive care unit (NICU), other diagnostic tools are used for the evaluation of PH in the BPD patients. Electrocardiography (ECG) and echocardiography may be used as screening methods for PH in most centers with NICU. Although ECG can be easily performed, it is known to have low sensitivity and positive predictive value for the diagnosis of RV hypertrophy in the precordial leads reflecting PH. In a recent study, Puchaiski et al. also showed no significant relationship between ECG findings and echocardiographic estimation of pulmonary artery pressure17). Therefore, serial echocardiography, in spite of its several limitations, is recommended as the main screening tool for PH in the BPD patients in many centers. In Seoul National University Children's Hospital, we conduct monthly echocardiographic examination of the preterm infants with BPD in the NICU to screen for PH and of the follow-up patients with moderate to severe BPD in the cardiologic division after discharge from the hospital even though the patients did not have PH in the NICU. A report from Boston Children's Hospital recommended that echocardiographic screening for PH should be conducted in preterm infants with BPD who meet any of the following criteria: (1) extreme prematurity (gestational age at birth of ≤25 weeks or birth weight of ≤600 g), (2) small for gestational age, (3) requirement for prolonged mechanical ventilatory support (duration depends on age), (4) oxygen requirement out of proportion to the severity of lung disease, or (5) persistent poor growth despite adequate caloric intake8).
Estimated systolic pulmonary artery pressure can be derived from the tricuspid regurgitant (TR) jet flow, which if present, is measured by echocardiography by using the modified Bernoulli equation [systolic pulmonary artery pressure=4×(TR peak jet velocity)2+right atrial pressure]18). However, estimation of systolic pulmonary artery pressure may not be possible in all the patients. A recent study revealed that estimation of systolic pulmonary artery pressure was possible in only 61% of the young children with PH19). In the absence of TR jet flow, other qualitative echocardiographic findings, including right atrial enlargement, RV hypertrophy and/or dilation, septal flattening, increased RV wall thickness, dilated main pulmonary artery, increased velocity of pulmonary valve regurgitation, and a short acceleration time of RV ejection into the pulmonary artery, can be used to detect PH; however, the sensitivity of these findings is questionable10, 12). Among these qualitative echocardiographic findings, interventricular septal configuration is very useful for evaluating the severity of PH in the BPD patients. Systolic RV pressure can be graded according to the position of the interventricular septum: (1) <50% of the systemic systolic pressure if the interventricular septum appears round at end-systole, (2) 50-100% of the systemic systolic pressure if the septum shows flattening at end-systole, and (3) ≥100% of the systemic systolic pressure if the interventricular septum bows into the left ventricle at end-systole (Fig. 1)20).
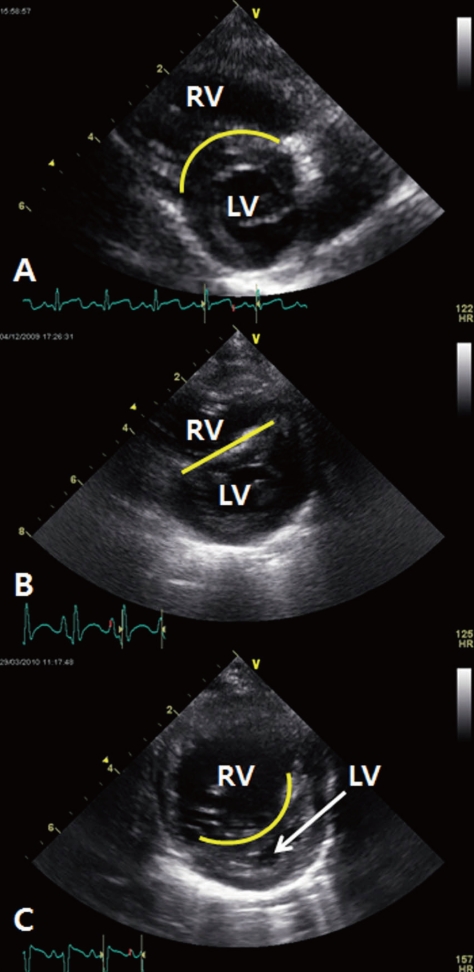
Interventricular septal configuration (IVS) for predicting the severity of pulmonary hypertension. (A), round IVS at end-systole, systolic right ventricular (RV) pressure is estimated to be less than 50% of the systemic systolic pressure. (B), flat IVS at end-systole, systolic RV pressure is estimated to be between 50 and 100% of the systemic systolic pressure. (C), IVS bowed into the left ventricle (LV) at end-systole, systolic RV pressure is estimated to be greater than 100% of the systemic systolic pressure.
Another useful parameter for the evaluation of PH severity is biochemical marker. B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) are representatives of biomarker and showed good positive correlations with mean pulmonary artery pressure, pulmonary vascular resistance and mean right atrial pressure. BNP also showed good negative correlation with RV ejection fraction21) even though normal value of children's age is not known well until now through extensive studies22). We have to be alert if the BPD patients with PH show high serum level or increasing level of BNP or NT-proBNP, because these parameters reflect disease status including severity.
In some centers, cardiac catheterization is generally recommended to evaluate the severity of PH, to rule out unsuspected associated anatomic cardiac diseases, to define the presence of systemic-to-pulmonary collateral vessels, pulmonary venous obstruction, or left heart dysfunction, and to assess pulmonary vascular reactivity in patients who fail to respond to oxygen treatment alone12).
Treatment of PH in the BPD patients
Most patients with PH showed gradual resolution of PH over the first year of life because the damaged lung recovered and normal alveolarization completed. However, because some BPD patients with severe PH could experience fatal right heart failure and other complications from PH6-9), meticulous and aggressive treatment of PH in the BPD patients is mandatory.
The basis of the treatment of PH in the BPD patients is to correct the underlying lung disease. These underlying conditions include chronic gastroesophageal reflux and aspiration, airway problems, such as laryngotracheomalacia, subglottic stenosis, and vocal cord palsy, and other conditions that cause intermittent or persistent hypoxia12). In case of repairable anatomic lesions to contribute to the PH, aggressive correction should be performed because the reduced vascular surface area in the BPD patients may result in significant hemodynamic injury caused by even a relatively small increase of left-to-right blood shunt through the patent foramen ovale, atrial septal defect, patent ductus arteriosus, or even other systemic-to-pulmonary collateral vessels12).
Oxygen supply is the mainstay of the medical treatment of PH in the BPD patients. Chronic hypoxia can induce pulmonary vascular constriction and aggravate PH, while sufficient oxygenation can be helpful in the recovery of damaged pulmonary tissue and normal growth of the lung. In this regard, oxygen saturation (SpO2 should be maintained at more than 93-95% in the patients with BPD23). The oxygen supply should be discontinued when the SpO2 is greater than 93-95% without oxygen supply. In particular, the BPD patients with PH should have an SpO2 in the mid-90%24, 25).
Besides oxygen supply, some BPD patients with PH require selective pulmonary vasodilators to decrease pulmonary arterial pressure; these medications subsequently decrease RV afterload and increase cardiac output from the RV. These actions result in an improvement of left ventricular filling and increase of systemic blood pressure and coronary perfusion26). The currently used medications for selective pulmonary vasodilation include inhaled nitric oxide (NO), inhaled prostacyclins, sildenafil, and endothelin-receptor antagonist. These medications can dilate the pulmonary artery and decrease the RV afterload through different mechanisms.
Inhaled NO selectively decreases pulmonary vascular resistance and increases systemic venous oxygen saturation by improving ventilation-perfusion match27). Inhaled NO improves oxygenation in infants with known BPD28) and may be useful for long-term treatment of pulmonary hypertension29). Although long-term inhaled NO treatment has been used in the BPD patients, especially those who require continuous mechanical ventilation, no study has reported such data to date. Preventive low-dose inhaled NO supply can reduce the incidence of BPD in the preterm infants with birth weight greater than 1,000 g, but it showed no effect in the infants with birth weight less than 1,000 g. Therefore, the use of preventive inhaled NO for BPD prevention is questionable30, 31). Most patients require an initial inhaled NO dose of 10-20 parts per million (ppm) for PH treatment and a maintenance dose of 2-10 ppm for weaning. Because sudden discontinuance of inhaled NO may cause rebound PH, other selective pulmonary vasodilators should be started before the discontinuance of inhaled NO even though the patient's condition is good32).
Inhaled prostacyclin is less expensive and more readily available than inhaled NO in the centers with NICU. Aerosolized prostacyclin may be administered directly to the lung, and it causes vasodilation in the ventilated pulmonary regions; it therefore minimizes the systemic side effects and helps avoid catheter-related complications in the patients33). Inhaled iloprost is the representative prostacyclin derivative; it easily dissolves in normal saline and has a lower viscosity than other prostacyclin derivatives34). The potential effect of inhaled iloprost for improving PH in the pediatric group has been established35). A case study had reported an improvement caused by inhaled iloprost in the BPD patient with severe PH36). For ensuring adequate improvement, inhalation should be performed 6-9 times a day because the half-life of the prostacyclin is extremely short. The occurrence of occasional bronchospasm may be a restricting factor in the use of inhaled prostacyclins by the BPD patients35).
Sildenafil, a highly selective phosphodiesterase type-5 inhibitor, increases cyclic GMP in the pulmonary vascular smooth muscle cells and decreases pulmonary vascular resistance. The effect of sildenafil has been well proven in the patients with RV failure caused by PH32). Sildenafil also has a synergistic effect with other pulmonary vasodilators, such as inhaled NO and inhaled iloprost, which have different pharmacologic mechanisms10, 37). A recent study on BPD patients with PH also showed that the use of sildenafil was safe and provided long-term beneficial effect38). The recommended starting dose of sildenafil is 0.5 mg/kg every 8 h. If the patient shows no systemic hypotension, the dose can be gradually increased to get the desired effect or up to 2 mg/kg every 6 h over 2 weeks12).
Endothelin-receptor antagonist inhibits the effect of endothelin-1, which is a potent vasoconstrictor, and induces pulmonary vascular smooth muscle cell hyperplasia12, 39). Bosentan is a representative non-selective endothelin-receptor antagonist that is orally administered. Bosentan improves exercise capacity of the patients with PH by decreasing RV dilatation and by improving RV function and left ventricular blood filling without significant negative effect on systemic pressure40, 41). Long-term randomized controlled studies have not been conducted in the patients with BPD using bosentan; however, other studies have shown that bosentan improved PH and functional status in daily life6, 42). The recommended starting dose of bosentan is 1.5 mg/kg/L every 12 h. If the patient shows no hepatic dysfunction after 2 weeks, the dose may be increased up to 3 mg/kg/d every 12 h. During the treatment, regular liver function tests must be conducted because bosentan is known to be hepatotoxic43).
Combination therapy, which involves the simultaneous prescription of more than one selective PH drug, has become the standard treatment in many centers. Several case series have suggested that various drug combinations appear to be safe and effective10); however, long-term safety and efficacy have not been evaluated in the pediatric population.
The advancements in pharmaceutical research will lead to the development of new selective pulmonary vasodilators in the near future.
Discontinuance of medications used for treating PH in the BPD patients should be determined only after serial echocardiography. If 2 consecutive serial echocardiographic examinations reveal normal or near-normal findings, weaning and discontinuance of medication for PH may be considered12).
Conclusions
Because of an increase in the number of preterm infants and a decrease in the gestational age at birth, more patients are showing significant BPD and secondary PH. PH contributes significantly to the high morbidity and mortality in the BPD patients. To prevent PH and to ensure early treatment if PH is present, regular monitoring for PH with echocardiography and biochemical markers like BNP or NT-proBNP is necessary in the BPD patients with greater than moderate degree. In the BPD patients with significant PH, multi-modality treatment, including treatment for correcting the underlying disease, oxygen supply, the use of diverse selective pulmonary vasodilators, and other methods, is mandatory.