Enterovirus 71-associated hand, foot and mouth diseases with neurologic symptoms, a university hospital experience in Korea, 2009
Article information
Abstract
Purpose
Hand-foot-mouth disease (HFMD) is a common viral illness in children, which is usually mild and self-limiting. However, in recent epidemics of HFMD in Asia, enterovirus 71 (EV71) has been recognized as a causative agent with severe neurological symptoms with or without cardiopulmonary involvement. HFMD was epidemic in Korea in the spring of 2009. Severe cases with complications including death have been reported. The clinical characteristics in children with neurologic manifestations of EV71 were studied in Ewha Womans University Mokdong Hospital.
Methods
Examinations for EV71 were performed from the stools, respiratory secretion or CSF of children who presented neurologic symptoms associated with HFMD by realtime PCR. Clinical and radiologic data of the patients were collected and analyzed.
Results
EV71 was isolated from the stool of 16 patients but not from respiratory secretion or CSF. Among the 16 patients, meningitis (n=10) was the most common manifestation, followed by Guillain-Barré syndrome (n=3), meningoencephalitis (n=2), poliomyelitis-like paralytic disease (n=1), and myoclonus (n=1). Gene analysis showed that most of them were caused by EV71 subgenotype C4a, which was prevalent in China in 2008.
Conclusion
Because EV71 causes severe complications and death in children, a surveillance system to predict upcoming outbreaks should be established and maintained and adequate public health measures are needed to control disease.
Introduction
Hand-foot-mouth disease (HFMD) is a common acute viral illness in children, which is usually mild and self-limiting. It is characterized by fever, oral ulcers, and vesicular exanthema on the hands, feet and buttocks. However, in recent epidemics of HFMD in Asia, severe cases with complications have been reported. Enterovirus 71 (EV71) has been recognized as a causative agent of HFMD with high neurological diseases and cardiopulmonary involvement1, 2). HFMD was epidemic in Korea in the spring of 2009. Severe cases with complications including death have been reported in HFMD patients, thus it has become a public health issue.
On April 2009, EV71 was isolated from the stool of a patient with poliomyelitis-like paralytic disease. From that time to July 2009, we have studied EV71 as a causative agent of HFMD in children with neurologic manifestations who were admitted to Ewha Womans University Mokdong Hospital. Examinations for EV71 were performed from the stools, respiratory secretion or CSF of children who presented neurologic symptoms associated with HFMD by realtime PCR.
Although this study is about a university hospital's experience of EV71-associated HFMD with neurologic symptoms, it is of great importance because there are few reports about EV71 infection in Korea.
Materials and methods
The subjects studied were 16 children hospitalized for neurologic symptoms with hand-foot-mouth disease at Ewha Womans University Mokdong Hospital, Seoul, Korea from April to July, 2009. Their ages ranged from a month to eight years.
Examinations for the viruses were carried out with stools, respiratory secretion or cerebrospinal fluid collected from the patients at the Division of Enteric and Hepatitis Viruses, Korea Centers of Diseases Control and Prevention. Clinical and radiologic data of the patients were collected and analyzed.
EV71 was detected by realtime PCR. Viral RNA was extracted using ZR Viral RNA Kit (Zymo Research, CA, USA) according to the manufacturer's instructions. The whole genomic RNA was reverse-transcribed for cDNA synthesis. Amplicons of the VP1 genes were generated using primers as follows: forward primer SO224 (5'-GCIATGYTIGGIACICAYRT-3') and reverse primer SO222 (5'-CICCIGGIGGIAYRWACAT-3') (1st-PCR); and forward primer AN89 (5'-CCAGCACTGACAGCAGYNGARAYNGG-3') and reverse primer AN88 (5'-TACTGGACCACCTGGNGGNAYRWACAT-3') (2nd-PCR). These PCR products were then sequenced using the ABI PRISM dye terminator cycle sequencing ready reaction kit (Perkin Elmer, Waltham, Massachusetts, USA), and run on an ABI 3100 sequencer (Applied Biosystems, Foster City, CA, USA). Nucleotide sequences were determined by DNASTAR.
Results
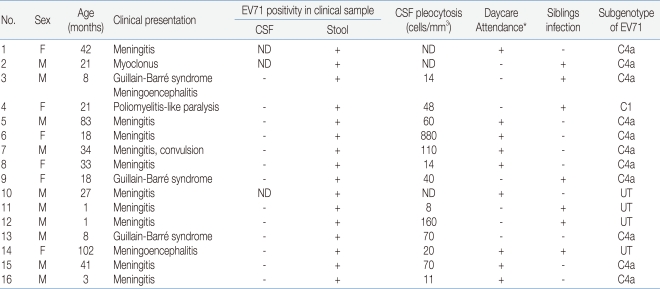
During the four months, 16 patients were hospitalized with neurologic manifestations related with HFMD (Table 1). Their ages ranged from a month to eight years and the mean age was 29 months. The male to female ratio was 1.7:1 (M:F).
Fever resolved in 2 to 7 days (mean, 4.5 days) and the range of peak of fever was 38.0 to 40.1℃ (mean, 39.3 ℃). In most of the patients, neurologic symptoms developed between day 2 and day 3 (range, day 1 to day 5) after the onset of fever or skin lesions.
Among the 16 patients, the most frequent neurologic manifestation was meningitis (62.5%), which included 8 definite cases with meningeal irritative symptoms and signs with CSF pleocytosis (>5 cells/mm3 and 2 suspected cases in whom spinal tappings were not performed. Three cases had Guillain-Barré syndrome and one of them had meningoencephalitis. One patient presenting asymmetric lower limb weakness (left>right) after HFMD showed enhancement in the left anterior horn of the spinal cord (T11 level) on magnetic resonance image.
EV71 were detected in the 16 patients presenting neurologic manifestations by realtime PCR. Clinical samples included stools, respiratory secretion, or cerebrospinal fluid obtained from the patients, but viruses were detected from only stools. Subgenotypes of enteroviruses were C1 in the poliomyelitis-like paralytic disease patient, and C4a in eleven patients presenting Guillain-Barré syndrome, meningitis or myoclonus.
Among the patients, only one girl presenting poliomyelitis-like paralytic disease still had limping when she was running at three months after discharge, but no death or severe complication with serious disease was observed in any of the other patients.
Discussion
We experienced 16 patients with EV71 who presented neurologic symptoms associated with HFMD from April to July, 2009. Most of them were diagnosed as meningitis or meningoencephalitis, and Guillain-Barré syndrome, poliomyelitis-like paralytic disease and myoclonus were other clinical diseases caused by EV71. There was no mortality, and one patient with poliomyelitis-like paralytic disease had sequelae. Gene analysis showed that most of them were caused by EV71 subgenotype C4a, which was prevalent in China in 2008.
HFMD, a benign and common febrile viral disease in childhood, is caused most frequently by coxsackievirus A16 and can also be caused by EV71, other coxsackieviruses and echoviruses. It is usually a mild illness, but in outbreaks caused by EV71, high incidences of severe complications including brainstem encephalitis, acute flaccid paralysis, neurogenic pulmonary edema, pulmonary hemorrhage, shock, and rapid death have been reported especially in children under 5 years of age3, 4).
Since EV71 was discovered in California in 1969, numerous outbreaks have occurred worldwide in countries of Europe, America, and Asia5-9). There have been several large epidemics of EV71 infection causing high fatalities. The first report of severe epidemics of encephalitis and acute flaccid paralysis due to EV71 came from Bulgaria in 1975, in which 44 deaths occurred10). In 1978, 45 patients died of neurologic diseases with HFMD in Hungary11). Since 1997, three major epidemics have occurred in the Asia-Pacific region. They were in Sarawak, Malaysia in 1997, Taiwan in 1998, and China in 2008, and 31, 78, and 126 fatal cases were reported, respectively1, 12, 13). In these EV71 outbreaks, the majority of affected children had HFMD or herpangina, but children having only febrile illness without mucocutaneous manifestations also had neurologic complications14). In this study, all the patients suspected to have neurologic diseases associated with EV71 had HFMD as a premonitory symptom, but none of them had herpangina. Approximately 10% of children with EV71-associated neurological disease in Taiwan had an initial or concurrent diagnosis of herpangina15).
Neurologic disorders have been prominent in recent epidemics of EV71 disease. In the outbreaks in Taiwan in 1998 and in Australia in 1999, virological studies found that HFMD and herpangina epidemics were caused by EV71 and coxsackievirus A16 but the cases presenting with acute pulmonary edema and other neurological diseases were caused by brainstem encephalitis resulting from acute EV71 infection4, 13, 16). Neurologic syndromes observed in EV71 include meningitis, meningoencephalomyelitis, poliomyelitis-like paralytic disease, Guillain-Barré syndrome, transverse myelitis, cerebellar ataxia, opsoclonus-myoclonus syndrome, benign intracranial hypertension, and brainstem encephalitis7, 16, 17). In this study, the patients showed variable manifestations in the spectrum of neurological disease and most of them recovered without sequelae after conservative management. The more varied presentations and milder clinical manifestations are likely to reflect the contribution of immunopathological process in addition to cytopathic damage to neuronal cells7, 18, 19). And, individual host genetic factors may affect clinical severity.
HFMD with EV71-associated neurological syndrome has increased continually in many countries during the last 10 years, especially in the Asia- Pacific region20, 21). In some countries, outbreaks occur in a cyclical pattern every three years, predominantly caused by strains that are distinct from previous outbreaks22). In Singapore, since the first outbreak of HFMD in 1970, many epidemics have occurred by predominantly circulating strain coxsackievirus A16 or EV71, and the largest one was caused by EV71 with 3,790 cases and four deaths in 20003, 23). In 2006, Brunei reported its first major outbreak of EV71 infection. In the outbreak, 1,681 children were affected with three deaths resulting from severe neurologic disease24). In the same year, an outbreak of EV71 affected approximately 14,400 children in Sarawak, Malaysia25). In the outbreak of China in 2008, a total of 488,955 HFMD cases were reported including 126 fatal cases, mostly caused by EV711). Possible reasons suggested for the outbreaks were mutation of the virus with increased virulence and the presence of host factors including the accumulation of susceptible populations and individual genetic susceptibility26, 27).
As it has been reported that some serious cases causing death or severe neurologic sequelae were associated with the EV71 infection in Korea in 2009, EV71 infection has become an important issue in public health. In 1990, 1993, 1997 and 2000, EV71 was reported as a causative agent of aseptic meningitis and poliomyelitis-like paralysis in Korea28-31). Since 2003, a sentinel surveillance system for enterovirus infection has been established in Korea. According to the surveillance system, 82 cases were reported in 2003, 36 in 2004, 404 in 2005, 251 in 2006, and 742 in 2008. Echoviruses and coxsackie viruses are major organisms causing aseptic meningitis.
In recent epidemics in the Asia-Pacific region, the predominant EV71 genotypes were various. In Sarawak during 1997 and Singapore during 1998, genotype B3 was most prevalent. EV71 isolated from patients in the Taiwanese epidemic during 1998 belonged to genotype C232, 33). During the epidemics in Malaysia and Singapore in 2000, the predominant EV71 genotype was B433). And, the EV71 outbreaks in Brunei, Sarawak and Singapore in 2006 were caused by subgenogroup B5 virus24). EV71 in the outbreak in China during 2007-2008 belonged to subgenotype C4a1, 20). The increasing fatality of EV71 infection in recent epidemics has been thought to be due to both endemic and epidemic circulation of the virus and its evolution. The possible occurrence of inter-typic recombination of EV71 may play important roles in the emergence of various EV71 subgenotypes with varied virulence and clinical manifestations34).
EV71 isolated from 12 patients during 2000 in Korea belonged to genotype C3, which has been identified rarely in other epidemics in recent years31, 32). As in Chinese strains in 2008-2009, the most frequent genotype among the EV71 strains isolated from our patients was C4a. Interestingly, a single isolate, which was from a girl with poliomyelitis-like paralysis, belonged to genotype C1. Genotype C1 viruses were isolated from cases of uncomplicated HFMD in Singapore and Sarawak during 1998 and in Sarawak and Western Australia during 2000, and appeared to have reduced neurovirulence6, 33).
It does not appear that a single neurovirulent genotype is associated with the severe and fatal cases as at least three distinct genotypes were isolated from fatal cases in Sarawak, Peninsular Malaysia, Japan and Taiwan7). However, further study is needed to elucidate the factors influencing the virulence of EV71.
The transmission of enterovirus occurs within families, daycare centers, playgrounds and hospital nurseries. All the patients who we studied attended daycare centers including kindergartens and schools by themselves, or had siblings with HFMD. More children would be congregating in a limited space, which provides a readily available reservoir for the rapid circulation of the virus3).
The fecal-to-oral route is considered a major transmission route for enteroviruses. Long periods of viral shedding may account for the widespread transmission of EV7135). Chung et al36) demonstrated that EV71 is excreted through the stool of infected patients for up to 6 weeks. Chang et al4) presented that the culture-positive rate of throat swabs was higher than that of rectal swabs in the patients. Viruses in throat through the saliva or respiratory droplets of patients may be transmitted during the acute stage of the disease37). Although hand-washing precaution is important for the period of virus excretion through feces, it is not sufficient to limit the spread and transmission of the virus and to prevent further epidemics. Therefore, isolation of infected patients within single rooms with masks should be considered35).
Public health measures, in particular, personal and environmental hygiene must target daycare centers, kindergartens, and schools where highly susceptible children congregate. In Singapore, to stem the spread of infection, preschools where transmission persisted for more than 2 incubation periods have been recommended to close, and trigger criteria for voluntary closure were instituted in April 2008. During closure, operators are to clean the centers thoroughly before they are allowed to reopen. In addition, parents are advised to ensure that their children adopt high-standard personal hygiene and to keep infected children at home until full recovery3).
In June 2009, Korea added HFMD and enterovirus infection to its list of nationally notifiable infectious diseases, for which all diagnosed cases must be reported within 7 days. This is expected to allow a more accurate and extensive survey of enterovirus infections nationwide and to lead to continuous collection of data about the incidence of EV71 infection and circulating viruses in Korea.
In conclusion, although HFMD is usually a benign disease, severe neurologic and cardiopulmonary complications can be developed in cases caused by EV71. In the Korean epidemic in 2009, these complications including death were reported and most of them were caused by EV71 subgenotype C4a. Because the outbreaks occur in a cyclical pattern, we need to establish a surveillance system to predict upcoming outbreaks and to develop adequate public health measures to control the outbreaks.