< Previous Next >
Article Contents
| Clin Exp Pediatr > Volume 57(10); 2014 |
|
Abstract
Adrenal and thyroid hormones are essential for the regulation of intrauterine homeostasis, and for the timely differentiation and maturation of fetal organs. These hormones play complex roles during fetal life, and are believed to underlie the cellular communication that coordinates maternal-fetal interactions. They serve to modulate the functional adaptation for extrauterine life during the perinatal period. The pathophysiology of systemic vasopressor-resistant hypotension is associated with low levels of circulating cortisol, a result of immaturity of hypothalamic-pituitary-adrenal axis in preterm infants under stress. Over the past few decades, studies in preterm infants have shown abnormal clinical findings that suggest adrenal or thyroid dysfunction, yet the criteria used to diagnose adrenal insufficiency in preterm infants continue to be arbitrary. In addition, although hypothyroidism is frequently observed in extremely low gestational age infants, the benefits of thyroid hormone replacement therapy remain controversial. Screening methods for congenital hypothyroidism or congenital adrenal hyperplasia in the preterm neonate are inconclusive. Thus, further understanding of fetal and perinatal adrenal and thyroid function will provide an insight into the management of adrenal and thyroid function in the preterm infant.
Adrenal and thyroid gland hormones are essential for the regulation of intrauterine homeostasis and timely differentiation and maturation of fetal organs; these hormones provide the cellular communications that coordinate maternal-fetal interactions1,2,3). During the perinatal period, they serve to modulate functional adaptations for extrauterine life4).
Over recent decades, the survival rate of preterm infants has improved, yet preterm infants continue to present with abnormal thyroid and cortisol axes. Infants with endocrine abnormalities are at an increased risk of abnormal development and morbidity5,6). As the physiology of preterm infants differs from that of term infants and older children, and normal physiological hormone levels of preterm infants at different gestational ages (GAs) remain unclear, no definitive management of endocrine problems in preterm infants has been determined as yet7,8,9,10,11).
This article reviews current understanding of the maturation of fetal adrenal and thyroid glands, and the roles of the adrenal and thyroid hormones during the infant's adaptation to extrauterine life; interpretation of clinical findings associated with these hormones in preterm infants is also discussed.
The fetal adrenal gland exhibits a remarkable transformation in size, morphology, and function during the fetal and perinatal period. In contrast to the adrenal medulla, which is derived from the neuroectoderm, the adrenal cortex is of mesodermal origin. The primitive adrenal glands can be recognized by 3 to 4 weeks of gestation12,13). The fetal adrenal gland is composed of three functional zones: a fetal zone (FZ), a transitional zone, and an outer definitive zone. The FZ mainly produces androgens, the transitional zone contains enzymes for cortisol production, and the definitive zone produces mineralocorticoids. The FZs become well differentiated by 9 to 12 weeks of gestation, and are capable of active steroidogenesis12). The fetal adrenal gland grows rapidly; the combined glandular weight is approximately 8 g at term, at which time the FZ makes up about 80% of the mass of the gland, with a relative size that is 10 to 20 folds that of the adult adrenal gland12,14). Soon after birth, the fetal adrenal gland undergoes rapid involution due to the rapid disappearance of the FZ; in contrast, the definitive zone, which comprises an inner zona fasciculata and an outer zona glomerulosa, proliferates1,14).
The fetal adrenal gland expresses five steroidogenic enzymes: 17-hydroxylase and 17, 20-desmolase (CYP17 or P450c17), 21-hydroxylase (CYP21A2 or P450c21), cholesterol side-chain cleavage (CYP11A1 or P450scc), aldosterone synthase (CYP11B2 or P450c11), and 3β-hydroxysteroid dehydrogenase (3βHSD)12). Since the FZ has relatively high steroid sulfotransferase activity and low 3βHSD activity, the major steroid products of the fetal adrenal gland are dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S)14), and there is a limited amount of cortisol and aldosterone (Fig. 1). Fetal steroidogenesis is largely programmed to produce inactive products, and provide DHEA substrates for placental estrone and estradiol production14). There is complementary activity between the enzymes involved in steroid formation and transformation between the placental and fetal compartments15) (Figs. 1, 2).
Prior to 23 weeks gestation, the human fetal adrenal cortex is unable to produce cortisol de novo and normally does not do so until as late as 30 weeks gestation1,14,16). Near term, the fetal cortisol production rate in the blood per unit body weight is similar to that in the adult12,14). About two thirds of fetal cortisol is derived from the fetal adrenal glands, and one third is derived from placental transfer. Fetal cortisol is converted to cortisone through an 11β hydroxysteroid dehydrogenase (11βHSD) in fetal tissues, and by midgestation, levels of circulating cortisone are 4 to 5 folds higher than cortisol concentrations17,18).
Fetal steroidogenesis is regulated by the hypothalamic-pituitary-adrenal (HPA) axis. The adrenocorticotropic hormone (ACTH) feedback control system matures progressively during the second half of gestation and early neonatal period12).
The steroid hormones produced by the fetal adrenal gland play key roles in the maintenance of pregnancy, intrauterine homeostasis, fetal maturation, and the initiation of parturition14).
The primordium of the human thyroid, which is derived from the epithelium of the pharyngeal floor, is initially recognizable at 16 to 17 days of gestation12). The primitive stalk connecting the primordium with the pharyngeal floor elongates into the thyroglossal duct; cells from the lower portion of thyroglossal duct differentiate into thyroid tissue12).
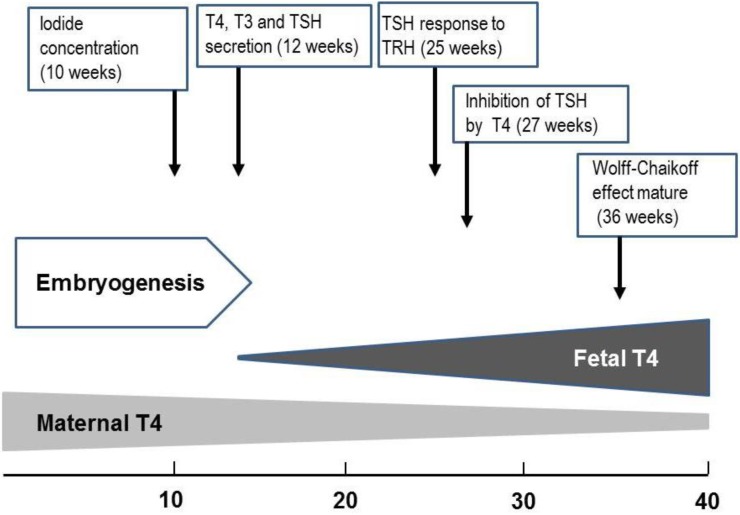
Embryogenesis of the human thyroid gland is largely completed by 10 to 12 weeks gestation, at which point tiny follicle precursors are visible19). Thyroid hormones are detectable in fetal serum by 12 weeks gestation; at that point, both thyroxine (T4) and triiodothyronine (T3) are measurable; however, a large proportion of detectable hormones derive from the mother through placental transfer20). During gestation, there is a gradual increase in the levels of thyroid hormones. While thyroglobulin (TG) can be identified in the fetal thyroid gland as early as the 5th week, maturation of TG secretion takes much longer21,22). While iodide concentrating capacity can be detected in the thyroid of the 10- to 11-week fetus, the capacity of the fetal thyroid gland to reduce iodide trapping in response to excess iodide does not appear until 36- to 40-week gestation23).
The fetus has detectable levels of thyroid-stimulating hormone (TSH) at GA 12 weeks. There is a moderate increase in TSH over the last two trimesters to levels of 6 to 8 mU/L at the time of delivery24). The fetal thyrotroph responds to thyrotropin-releasing hormone (TRH) as early as 25 weeks gestation24). The maturation of the negative feedback control of thyroid hormone synthesis occurs around midgestation23,24) (Fig. 3).
During gestation, circulating concentrations of T4 and the active metabolite T3 are low, while the inactive metabolites, reverse T3 (rT3) and T3 sulfate, are high. This pattern is a consequence of both immaturity of the hypothalamic-pituitary-thyroid axis, and coordinated adjustments in the deiodinase system. The level of type 1 iodothyronine deiodinase (D1), which catalyzes T4 to T3 conversion, is low throughout gestation. Levels of type 2 deiodinase (D2), which converts T4 to T3, and type 3 deiodinase (D3), an inactivating deiodinase that converts T4 to rT3, are high25). Despite low concentrations of circulating T3, by 20- to 26-week gestation T3 levels in the fetal brain are approaching 60%-80% of adult values. While the physiological significance of low circulating T3 concentrations throughout gestation is unknown, it has been suggested that its function may be to avoid tissue thermogenesis and potentiate the anabolic state of the rapidly growing fetus26).
The placenta produces various hormones that can influence the fetal thyroid gland. The most important role of the placenta, however, is in regulating the passage of hormones and drugs from the mother to the embryo, a process that influences the fetal thyroid gland (Fig. 2).
Rathke's pouch separates from the primitive pharyngeal stomodeum by 5-week gestation27). The bony floor of the sella turcica is present by 7 weeks of gestation. Intact hypothalamic-pituitary portal vessels are present by 12 to 17 weeks of gestation. Maturation of the pituitary portal vascular system continues, extending to 30- to 35-weeks of gestation12).
After delivery, the neonate must initiate breathing and defend against hypothermia, hypoglycemia, and hypocalcemia, as the placental supply of energy and nutrients are abruptly removed. Fetal hormones, especially from the adrenal cortex and thyroid gland, rapidly respond to these changes.
Human fetal cortisol levels tend to be as low as 5-10 µg/mL until about 30-week gestation. Cortisol levels increase progressively, reaching ~20 µg/mL by 36 weeks of gestation, and 45 µg/mL at term. Cortisol increases further during labor, peaking to levels of ~200 µg/mL several hours after term delivery4,28). This cortisol surge is mediated by a decrease in the conversion of cortisol to cortisone, with a simultaneous increase in cortisol production by the fetal adrenal gland. Cesarean section of the unlabored fetus blunts the postnatal rise in cortisol29); also, during preterm birth, the cortisol responses are attenuated because of the immaturity and unresponsiveness of the adrenal gland29).
The cortisol surge augments surfactant synthesis in lung tissue, increases reabsorption of liquid in the lung, increases methylation of norepinephrine to epinephrine, increases conversion of T4 to T3, facilitates ductus closure, induces maturation of several enzymes and transport processes of the small intestine, and stimulates maturation of hepatic enzymes12). Prenatal inflammation, as observed in chorioamnionitis, leads to adrenal stimulation, which results in increased cortisol secretion30,31).
During parturition, the neonate must rapidly convert from the fetal state of predominant thyroid hormone inactivation to a state of relative thyroid hyperactivity; this is initiated by an abrupt increase in hypothalamic TRH and pituitary TSH secretion. The cold-stimulated TRH-TSH surge is short-lived and peaks at 30 minutes, with peak concentrations as high as 60 to 70 µU/L32); thereafter, serum TSH concentrations progressively decrease to normal infant levels by 3 to 5 days, while serum-free T4 levels remain elevated for several weeks33).
While acute ablation of thyroid function at birth has not been shown to greatly alter thermogenesis or cardiovascular adaptation, chronic inhibition of thyroid function prior to birth has been shown to interfere with postnatal cardiovascular adaptation and thermogenesis in newborn lambs34). These results show that thyroid hormones play an important role in the preparation for birth, rather than in modulating endocrine adaptation to birth. Preterm infants have a blunted TSH surge, with very low levels of plasma T3 and T4, relative to term infants.
The main functions of the postnatal adrenal gland are to regulate protein, carbohydrate, lipid, and nucleic acid metabolism; maintain vascular responsiveness to circulating vasoconstrictors; oppose the increase in capillary permeability during acute inflammation; regulate extracellular water by reducing movement of water into cells and promoting water excretion; suppress the inflammatory response; and modulate central nervous system processing and behavior5).
Activation of the HPA axis is crucial in maintaining homeostasis in response to stress; otherwise, the preterm infant would have limited ability to maintain postpartum homeostasis. Developmental immaturity and illness-induced adrenal insufficiency may contribute to inadequate adrenal function. In preterm infants, adrenal cortex function is closely related to the duration of gestation35). However, in preterm infants of less than 30-week GAs, the cortisol production rate, assessed by urinary cortisol metabolites, approaches the cortisol production rate of older children and adults. The surge in cortisol production is absent in preterm infants during clinical illness36). Although the human fetal adrenal cortex does not express the 3βHSD enzyme prior to 23-week gestation, there is no evidence of significant immaturity in adrenal 3βHSD activity in preterm infants born between 24-28 weeks of gestation37). Blood concentrations of cortisol and other steroid hormones are no lower in preterm infants with late onset adrenal insufficiency than in control preterm infants38). These findings suggest that while preterm infants might not have an absolute deficiency of cortisol production, their ability to synthesize sufficient cortisol for the corresponding degree of clinical stress may be limited.
Activation of the HPA axis is crucial in maintaining homeostasis in response to stress. While there is no evidence of clinical adrenocortical insufficiency in term infants, clinically ill and preterm infants may have limited ability to produce adequate amounts of glucocorticoids.
Systemic hypotension is a common complication in sick preterm infants. While the cause of hypotension in the preterm infant is multifactorial, multiple studies on extremely low birth weight infants have demonstrated that hypotension responds to glucocorticoids, while being refractory to volume expanders and vasopressors39,40). Recent studies have demonstrated low levels of circulating cortisol in preterm infants under stress, suggesting that the pathophysiology of systemic hypotension is associated with the immaturity of the HPA axis36,38).
Transient adrenocortical insufficiency of prematurity (TAP) is the term used to describe the clinical scenario wherein preterm newborns in the immediate postnatal period have normal or enhanced pituitary response; however, their adrenal glands have a transient inability to maintain cortisol homoeostasis41,42). TAP is frequently associated with systemic hypotension and results from an immature HPA axis, and reduced ability of the adrenal glands to produce cortisol in response to deficiencies of intermediate enzymes in the synthesis pathway, such as 11β-hydroxylase41,42). TAP is typically transient, and adrenal function tends to return to normal by 2-week postpartum. Therefore, glucocorticoid-responsive hypotension is not considered a common phenomenon in this population beyond 2-week postpartum. However, preterm infants sometimes develop late-onset glucocorticoid-responsive circulatory collapse38). The pathophysiology of late-onset adrenal insufficiency in preterm infants (AIP) is not due to an absolute deficiency of cortisol production; instead, it may be due to a limited ability to synthesize sufficient cortisol for the corresponding degree of clinical stress38). Clinical predictors of AIP include hypotension, oliguria, hyponatremia, lung edema, increased demand for oxygen in the absence of infection, hypovolemia, anemia, and the reopening of a patent ductus arteriosus.
There are no definitive diagnostic criteria for AIP. A presumptive diagnosis can be made in case the clinical picture indicates adrenal insufficiency, inappropriately low serum cortisol levels, and rapid recovery from signs of adrenal insufficiency following cortisol replacement. A serum cortisol level of <15 µg/dL is frequently used for diagnosis of AIP. This level was based on relative adrenal insufficiency in critically ill adults and a study of critically ill term neonates that demonstrated improvement in hemodynamic parameters with hydrocortisone therapy, selectively in patients with initial cortisol concentrations of <15 µg/dL43,44). A cortisol increase of <9 µg/dL in response to low dose adrenocorticotropin (ACTH) stimulation (1 µg/kg of synthetic ACTH) is also used for the diagnosis of AIP. However, in preterm infants, neither baseline cortisol <15 µg/dL nor Δ-cortisol <9 µg/dL were associated with the presence of relative adrenal insufficiency between days 5 and 7 postpartum45). Some authors have recommended measuring the cortisol levels in serum or saliva in response to a corticotropin releasing hormone (CRH) test (1 µg/kg of hCRH) as a reliable method to evaluate the HPA axis in the preterm infant46,47).
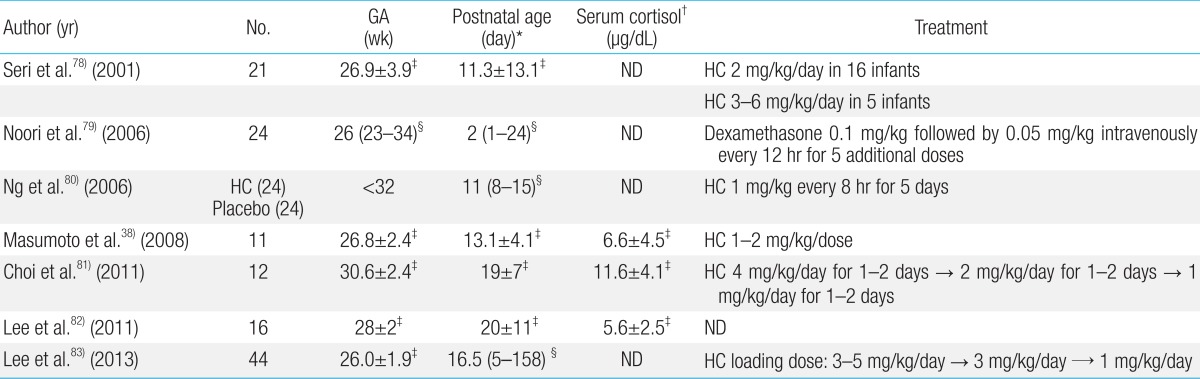
Hydrocortisone is greatly preferred over dexamethasone for treatment of AIP, because it has a limited effect on suppression of growth, and influences both glucocorticoids and mineralocorticoids. Various dosages and durations of hydrocortisone therapy have been used for replacement of AIP (Table 1). Further studies are warranted in order to verify the diagnostic criteria and optimal treatment of AIP.
Congenital adrenal hyperplasia (CAH), which is caused by 21-hydroxylase deficiency, is an inherited metabolic disorder that affects 1 per 16,000 neonates48,49). Mass screening of neonates for 21-hydroxylase deficiency identifies both male and female infants, prevents incorrect sex assignment, and decreases mortality and morbidity due to salt-wasting crisis. Most newborn screening programs measure 17-hydroxyprogesterone (17-OHP) from dried blood spots on filter paper; however, 17-OHP measurement has a high false positive rate in preterm infants50).
The mechanisms underlying high 17-OHP concentrations in preterm infants are unclear, because 21-hydroxylase is actively expressed during early midgestation and 3βHSD is expressed during late midgestation1). Possible explanations for the increased levels of 17-OHP in preterm infants include an increase in the conversion of cholesterol to pregnenolone due to increased ACTH from postnatal stress51); decrease in conversion of 11-deoxycortisol to cortisol due to delayed expression of 11β-hydroxylase52); and decrease in the excretion of steroid metabolites in the kidney50). Another probable explanation is that there is cross-reactivity while measuring 17-OHP, with other steroid metabolites such as 17-hydroxypregnenolone and its sulfated metabolites53,54).
Conversely, antenatal corticosteroid administration can interfere with CAH screening programs, because corticosteroids are known to suppress the HPA axis55,56). Since betamethasone and dexamethasone are similar in their ability to cross the placenta and suppress the fetal pituitary-adrenal axis, the use of antenatal corticosteroids may increase the risk of lowering 17-OHP levels in the blood spot, thus leading to false-negative results.
While screening preterm infants for CAH, the rates for false positive and false negative results are high; however, there is a low risk of missing a case of CAH that might lead to a salt-wasting crisis in the neonatal intensive care unit. Rescreening of preterm infants with elevated 17-OHP levels, with careful monitoring of the clinical status during intervals, is recommended57).
Postnatal thyroid function of preterm infants differs from that of term infants. Blunted postnatal TSH surges and low serum T4 levels are frequently observed in preterm neonates; this is generally referred to as hypothyroxinemia of prematurity58). In contrast to typical congenital hypothyroidism, initial screening indicates a normal TSH level, followed by delayed TSH elevation in some preterm infants59).
The main factors that influence thyroid function in preterm infants are immaturity of the hypothalamic-pituitary-thyroid axis, immature thyroid hormone synthesis, immature thyroid hormone metabolism, and systemic diseases. Insufficient or excessive iodine intake also influence preterm thyroid function60).
Transient hypothyroxinemia of prematurity (THOP) is a condition that primarily affects preterm infants born at less than 30 weeks of gestation, and is characterized by low levels of circulating thyroid hormones despite normal levels of TSH58).
A blunted TSH surge after birth is one of the reasons for low T4 levels in the preterm infant61). The other reason is reduced storage of iodine, which can exist due to prematurity20,62). In addition, very low birth weight infants usually have various systemic diseases and are given drugs such as dopamine, dobutamine, and morphine that affect the hypothalamic-pituitary-thyroidal axis. Thus, TSH levels are not representative of overall thyroid function in preterm infants.
The depth of the nadir and length of time before THOP resolves is related to GA. This condition usually resolves within 2 to 3 weeks, with progressive maturation of the hypothalamic-pituitary-thyroid axis63). Although no consensus exists for THOP reference ranges, prevalence rates have been reported to be 35%-85% in very preterm infants64).
Although transiently low levels of thyroid hormones are associated with higher rates of cerebral palsy and cognitive impairment in preterm infants, studies have not demonstrated the benefits of thyroid hormone replacement (Table 2). In a meta-analysis, prophylactic thyroid hormone replacement in preterm infants was not shown to be beneficial in reducing neonatal mortality or morbidity, or in improving neurodevelopmental outcomes65).
The incidence of persistent hypothyroidism does not differ among preterm and term newborns; however, transient hypothyroidism is considerably more prevalent59). The estimated incidence of delayed TSH elevation is up to 12% in preterm infants63,64). Although the timing of this elevation varies, it usually develops between 2 and 6 weeks of age in most cases. Although the reasons for delayed TSH elevation in the preterm infant may be complex, iodine deficiency or excess are the likely reasons for transient hypothyroidism in the preterm infant. The daily iodine requirement of preterm infants is more than twice that of term infants66), and studies conducted in Europe have demonstrated that most preterm infants have iodine deficiency67,68,69). On the other hand, iodine excess is associated with delayed TSH elevation in the preterm infant70,71). Since sodium/iodide symporters are expressed in the mammary gland, excessive iodine in the lactating mother can be directly transferred to the infant72). The skin of preterm infants is thin and may absorb iodine easily, and preterm infants have many opportunities of exposure to iodine-containing disinfectants73). Since downregulation of the sodium/iodide symporter (i.e., escape from the Wolff-Chaikoff effect) does not occur in the fetus until the third trimester and seems to appear at >35-week GA in preterm infants12), thyroid function in preterm infants is vulnerable to excessive iodine intake. Dopamine is known to suppress thyrotropin release, and transfusion may affect thyroid function test results74).
As routine neonatal screening for congenital hypothyroidism may fail to detect the atypical form of hypothyroidism with delayed TSH elevation, recent screening guidelines recommend repeated screening in the preterm infant75,76). The repeat specimen should be collected at either 2 weeks of age, or 2 weeks after the first screening test was carried out. However, repeat screening has not been adopted by all screening programs, because elevated TSH is mostly a transient problem77). Further studies on the etiology and developmental outcomes of delayed TSH elevation are needed for better clinical practice.
Adrenal and thyroid hormones play various roles in somatic development and maintenance of homeostasis throughout the fetal and neonatal periods. Whereas abnormal clinical findings associated with adrenal or thyroid dysfunction are not rare in preterm infants, the diagnostic criteria and optimal management have not been determined yet. Further understanding of fetal and perinatal adrenal and thyroid function will enhance clinician insight into the management of adrenal and thyroid dysfunction in the preterm infant. Further research is required to improve the understanding of the pathophysiology and management of adrenal and thyroid dysfunction in the preterm infant.
References
1. Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev 1997;18:378–403.


2. Fisher DA. Fetal thyroid function: diagnosis and management of fetal thyroid disorders. Clin Obstet Gynecol 1997;40:16–31.


3. Ng PC. The fetal and neonatal hypothalamic-pituitary-adrenal axis. Arch Dis Child Fetal Neonatal Ed 2000;82:F250–F254.



4. Hillman NH, Kallapur SG, Jobe AH. Physiology of transition from intrauterine to extrauterine life. Clin Perinatol 2012;39:769–783.



5. Watterberg KL. Adrenocortical function and dysfunction in the fetus and neonate. Semin Neonatol 2004;9:13–21.


6. La Gamma EF, van Wassenaer AG, Ares S, Golombek SG, Kok JH, Quero J, et al. Phase 1 trial of 4 thyroid hormone regimens for transient hypothyroxinemia in neonates of <28 weeks' gestation. Pediatrics 2009;124:e258–e268.



7. Heckmann M, Wudy SA, Haack D, Pohlandt F. Reference range for serum cortisol in well preterm infants. Arch Dis Child Fetal Neonatal Ed 1999;81:F171–F174.



8. Heckmann M, Hartmann MF, Kampschulte B, Gack H, Bodeker RH, Gortner L, et al. Cortisol production rates in preterm infants in relation to growth and illness: a noninvasive prospective study using gas chromatography-mass spectrometry. J Clin Endocrinol Metab 2005;90:5737–5742.


9. Ng PC. Is there a "normal range" of serum cortisol concentration for preterm infants? Pediatrics 2008;122:873–875.


10. Calixto C, Martinez FE, Jorge SM, Moreira AC, Martinelli CE Jr. Correlation between plasma and salivary cortisol levels in preterm infants. J Pediatr 2002;140:116–118.


11. Williams FL, Simpson J, Delahunty C, Ogston SA, Bongers-Schokking JJ, Murphy N, et al. Developmental trends in cord and postpartum serum thyroid hormones in preterm infants. J Clin Endocrinol Metab 2004;89:5314–5320.


12. Kronenberg HM, Melmed S, Polonsky KS, Larsen PR. Williams textbook of endocrinology. 11th ed. Philadelphia: Saunders, 2008.
13. Hanley NA, Rainey WE, Wilson DI, Ball SG, Parker KL. Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Mol Endocrinol 2001;15:57–68.


14. Ishimoto H, Jaffe RB. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr Rev 2011;32:317–355.


15. Pasqualini JR. Enzymes involved in the formation and transformation of steroid hormones in the fetal and placental compartments. J Steroid Biochem Mol Biol 2005;97:401–415.


16. Rainey WE, Rehman KS, Carr BR. Fetal and maternal adrenals in human pregnancy. Obstet Gynecol Clin North Am 2004;31:817–835.


17. Benediktsson R, Calder AA, Edwards CR, Seckl JR. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf) 1997;46:161–166.


18. Murphy BE, Clark SJ, Donald IR, Pinsky M, Vedady D. Conversion of maternal cortisol to cortisone during placental transfer to the human fetus. Am J Obstet Gynecol 1974;118:538–541.


19. Ballabio M, Nicolini U, Jowett T, Ruiz de Elvira MC, Ekins RP, Rodeck CH. Maturation of thyroid function in normal human foetuses. Clin Endocrinol (Oxf) 1989;31:565–571.


20. Contempre B, Jauniaux E, Calvo R, Jurkovic D, Campbell S, de Escobar GM. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J Clin Endocrinol Metab 1993;77:1719–1722.


21. De Nayer P, Cornette C, Vanderschueren M, Eggermont E, Devlieger H, Jaeken J, et al. Serum thyroglobulin levels in preterm neonates. Clin Endocrinol (Oxf) 1984;21:149–153.


22. Sobrero G, Muñoz L, Bazzara L, Martin S, Silvano L, Iorkansky S, et al. Thyroglobulin reference values in a pediatric infant population. Thyroid 2007;17:1049–1054.


23. Krassas GE, Rivkees SA, Kiess W. Diseases of the thyroid in childhood and adolescence. Basel: S Karger AG, 2007.
24. Thorpe-Beeston JG, Nicolaides KH, Felton CV, Butler J, McGregor AM. Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N Engl J Med 1991;324:532–536.


25. Kester MH, Martinez de Mena R, Obregon MJ, Marinkovic D, Howatson A, Visser TJ, et al. Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas. J Clin Endocrinol Metab 2004;89:3117–3128.


26. Sperling MA. Pediatric endocrinology. 3rd ed. Philadelphia: Saunders, 2008.
27. Polk DH, Reviczky A, Lam RW, Fisher DA. Thyrotropin-releasing hormone in ovine fetus: ontogeny and effect of thyroid hormone. Am J Physiol 1991;260(1 Pt 1): E53–E58.


28. Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev 1994;6:141–150.


29. Bird JA, Spencer JA, Mould T, Symonds ME. Endocrine and metabolic adaptation following caesarean section or vaginal delivery. Arch Dis Child Fetal Neonatal Ed 1996;74:F132–F134.



30. Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97:210–215.



31. Watterberg KL, Scott SM, Naeye RL. Chorioamnionitis, cortisol, and acute lung disease in very low birth weight infants. Pediatrics 1997;99:E6


33. Fisher DA, Nelson JC, Carlton EI, Wilcox RB. Maturation of human hypothalamic-pituitary-thyroid function and control. Thyroid 2000;10:229–234.


34. Honour JH, Wickramaratne K, Valman HB. Adrenal function in preterm infants. Biol Neonate 1992;61:214–221.


35. Bolt RJ, Van Weissenbruch MM, Popp-Snijders C, Sweep FG, Lafeber HN, Delemarre-van de Waal HA. Maturity of the adrenal cortex in very preterm infants is related to gestational age. Pediatr Res 2002;52:405–410.


36. Fernandez EF, Montman R, Watterberg KL. ACTH and cortisol response to critical illness in term and late preterm newborns. J Perinatol 2008;28:797–802.



37. Nykanen P, Heinonen K, Riepe FG, Sippell WG, Voutilainen R. Serum concentrations of adrenal steroids and their precursors as a measure of maturity of adrenocortical function in very premature newborns. Horm Res Paediatr 2010;74:358–364.


38. Masumoto K, Kusuda S, Aoyagi H, Tamura Y, Obonai T, Yamasaki C, et al. Comparison of serum cortisol concentrations in preterm infants with or without late-onset circulatory collapse due to adrenal insufficiency of prematurity. Pediatr Res 2008;63:686–690.


39. Colasurdo MA, Hanna CE, Gilhooly JT, Reynolds JW. Hydrocortisone replacement in extremely premature neonates with cortisol insufficiency [abstract]. Clin Res 1989;37:180A
40. Ward RM, Kimura RE, Rich-Denson C. Addisonian crisis in extremely premature neonates [abstract]. Clin Res 1991;39:11A
41. Ng PC, Lam CW, Fok TF, Lee CH, Ma KC, Chan IH, et al. Refractory hypotension in preterm infants with adrenocortical insufficiency. Arch Dis Child Fetal Neonatal Ed 2001;84:F122–F124.



42. Ng PC, Lee CH, Lam CW, Ma KC, Fok TF, Chan IH, et al. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2004;89:F119–F126.



43. Fernandez E, Schrader R, Watterberg K. Prevalence of low cortisol values in term and near-term infants with vasopressor-resistant hypotension. J Perinatol 2005;25:114–118.


44. Langer M, Modi BP, Agus M. Adrenal insufficiency in the critically ill neonate and child. Curr Opin Pediatr 2006;18:448–453.


45. Sari FN, Dizdar EA, Oguz SS, Andiran N, Erdeve O, Uras N, et al. Baseline and stimulated cortisol levels in preterm infants: is there any clinical relevance? Horm Res Paediatr 2012;77:12–18.


46. Ng PC, Wong GW, Lam CW, Lee CH, Wong MY, Fok TF, et al. The pituitary-adrenal responses to exogenous human corticotropin-releasing hormone in preterm, very low birth weight infants. J Clin Endocrinol Metab 1997;82:797–799.


47. Matsukura T, Kawai M, Marumo C, Iwanaga K, Yoshida K, Shibata M, et al. Diagnostic value of salivary cortisol in the CRH stimulation test in premature infants. J Clin Endocrinol Metab 2012;97:890–896.


48. White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev 2000;21:245–291.


50. Nordenstrom A, Wedell A, Hagenfeldt L, Marcus C, Larsson A. Neonatal screening for congenital adrenal hyperplasia: 17-hydroxyprogesterone levels and CYP21 genotypes in preterm infants. Pediatrics 2001;108:E68


51. Huysman MW, Hokken-Koelega AC, De Ridder MA, Sauer PJ. Adrenal function in sick very preterm infants. Pediatr Res 2000;48:629–633.


52. Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA. Adrenal steroidogenesis in very low birth weight preterm infants. J Clin Endocrinol Metab 1994;78:266–270.


53. Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, Ekins S. Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol 201414;14:33
54. Riepe FG, Mahler P, Sippell WG, Partsch CJ. Longitudinal study of plasma pregnenolone and 17-hydroxypregnenolone in full-term and preterm neonates at birth and during the early neonatal period. J Clin Endocrinol Metab 2002;87:4301–4306.


55. Kari MA, Raivio KO, Stenman UH, Voutilainen R. Serum cortisol, dehydroepiandrosterone sulfate, and steroid-binding globulins in preterm neonates: effect of gestational age and dexamethasone therapy. Pediatr Res 1996;40:319–324.


56. Gatelais F, Berthelot J, Beringue F, Descamps P, Bonneau D, Limal JM, et al. Effect of single and multiple courses of prenatal corticosteroids on 17-hydroxyprogesterone levels: implication for neonatal screening of congenital adrenal hyperplasia. Pediatr Res 2004;56:701–705.


57. Chung HR, Shin CH, Yang SW, Yun KA, Lee YA, Park SE, et al. Interpretation of screening for congenital adrenal hyperplasia in preterm infants. Korean J Pediatr 2008;51:616–621.

58. Uhrmann S, Marks KH, Maisels MJ, Friedman Z, Murray F, Kulin HE, et al. Thyroid function in the preterm infant: a longitudinal assessment. J Pediatr 1978;92:968–973.


59. Mandel SJ, Hermos RJ, Larson CA, Prigozhin AB, Rojas DA, Mitchell ML. Atypical hypothyroidism and the very low birth-weight infant. Thyroid 2000;10:693–695.


60. van Wassenaer AG, Kok JH. Hypothyroxinaemia and thyroid function after preterm birth. Semin Neonatol 2004;9:3–11.


61. Frank JE, Faix JE, Hermos RJ, Mullaney DM, Rojan DA, Mitchell ML, et al. Thyroid function in very low birth weight infants: effects on neonatal hypothyroidism screening. J Pediatr 1996;128:548–554.


62. Savin S, Cvejie D, Nedic O, Radosavljevic R. Thyroid hormone synthesis and storage in the thyroid gland of human neonates. J Pediatr Endocrinol Metab 2003;16:521–528.

63. Chung HR, Shin CH, Yang SW, Choi CW, Kim BI, Kim EK, et al. High incidence of thyroid dysfunction in preterm infants. J Korean Med Sci 2009;24:627–631.



64. Scratch SE, Hunt RW, Thompson DK, Ahmadzai ZM, Doyle LW, Inder TE, et al. Free thyroxine levels after very preterm birth and neurodevelopmental outcomes at age 7 years. Pediatrics 2014;133:e955–e963.



65. Osborn DA, Hunt RW. Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2007;(1): CD005948


66. Delange F. Optimal iodine nutrition during pregnancy, lactation and the neonatal period. Int J Endocrinol Metab 2004;2:1–12.
67. Ares S, Escobar-Morreale HF, Quero J, Duran S, Presas MJ, Herruzo R, et al. Neonatal hypothyroxinemia: effects of iodine intake and premature birth. J Clin Endocrinol Metab 1997;82:1704–1712.


68. Ibrahim M, de Escobar GM, Visser TJ, Duran S, van Toor H, Strachan J, et al. Iodine deficiency associated with parenteral nutrition in extreme preterm infants. Arch Dis Child Fetal Neonatal Ed 2003;88:F56–F57.



69. van Wassenaer AG, Stulp MR, Valianpour F, Tamminga P, Ris Stalpers C, de Randamie JS, et al. The quantity of thyroid hormone in human milk is too low to influence plasma thyroid hormone levels in the very preterm infant. Clin Endocrinol (Oxf) 2002;56:621–627.


70. Chung HR, Shin CH, Yang SW, Choi CW, Kim BI. Subclinical hypothyroidism in Korean preterm infants associated with high levels of iodine in breast milk. J Clin Endocrinol Metab 2009;94:4444–4447.


71. Larson C, Hermos R, Delaney A, Daley D, Mitchell M. Risk factors associated with delayed thyrotropin elevations in congenital hypothyroidism. J Pediatr 2003;143:587–591.


72. De La Vieja A, Dohan O, Levy O, Carrasco N. Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev 2000;80:1083–1105.


73. Aitken J, Williams FL. A systematic review of thyroid dysfunction in preterm neonates exposed to topical iodine. Arch Dis Child Fetal Neonatal Ed 2014;99:F21–F28.


74. Van den Berghe G, de Zegher F. Anterior pituitary function during critical illness and dopamine treatment. Crit Care Med 1996;24:1580–1590.


75. Leger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab 2014;99:363–384.



76. Leger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm Res Paediatr 2014;81:80–103.


77. Woo HC, Lizarda A, Tucker R, Mitchell ML, Vohr B, Oh W, et al. Congenital hypothyroidism with a delayed thyroid-stimulating hormone elevation in very premature infants: incidence and growth and developmental outcomes. J Pediatr 2011;158:538–542.


78. Seri I, Tan R, Evans J. Cardiovascular effects of hydrocortisone in preterm infants with pressor-resistant hypotension. Pediatrics 2001;107:1070–1074.


79. Noori S, Siassi B, Durand M, Acherman R, Sardesai S, Ramanathan R. Cardiovascular effects of low-dose dexamethasone in very low birth weight neonates with refractory hypotension. Biol Neonate 2006;89:82–87.


80. Ng PC, Lee CH, Bnur FL, Chan IH, Lee AW, Wong E, et al. A double-blind, randomized, controlled study of a "stress dose" of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics 2006;117:367–375.


81. Choi EJ, Sohn JA, Lee EH, Lee JY, Lee HJ, Chung HR, et al. Clinical picture of adrenal insufficiency-associated hypotension in preterm infants. J Korean Soc Neonatol 2011;18:82–88.

82. Lee JA, Choi CW, Kim EK, Kim HS, Kim BI, Choi JH. Late-onset hypotension and late circulatory collapse due to adrenal insufficiency in preterm infants with gestational age less than 32 weeks. J Korean Soc Neonatol 2011;18:211–220.

83. Lee WJ, Kim MY, Cho HJ, Lee JS, Son DW. Clinical features of late-onset circulatory collapse in preterm infants. Korean J Perinatol 2013;24:148–157.

84. Chowdhry P, Scanlon JW, Auerbach R, Abbassi V. Results of controlled double-blind study of thyroid replacement in very low-birth-weight premature infants with hypothyroxinemia. Pediatrics 1984;73:301–305.



85. Amato M, Pasquier S, Carasso A, Von Muralt G. Postnatal thyroxine administration for idiopathic respiratory distress syndrome in preterm infants. Horm Res 1988;29:27–30.


86. Smith LM, Leake RD, Berman N, Villanueva S, Brasel JA. Postnatal thyroxine supplementation in infants less than 32 weeks' gestation: effects on pulmonary morbidity. J Perinatol 2000;20:427–431.


87. Biswas S, Buffery J, Enoch H, Bland M, Markiewicz M, Walters D. Pulmonary effects of triiodothyronine (T3) and hydrocortisone (HC) supplementation in preterm infants less than 30 weeks gestation: results of the THORN trial: thyroid hormone replacement in neonates. Pediatr Res 2003;53:48–56.


88. van Wassenaer AG, Kok JH, de Vijlder JJ, Briet JM, Smit BJ, Tamminga P, et al. Effects of thyroxine supplementation on neurologic development in infants born at less than 30 weeks' gestation. N Engl J Med 1997;336:21–26.


89. van Wassenaer AG, Briet JM, van Baar A, Smit BJ, Tamminga P, de Vijlder JJ, et al. Free thyroxine levels during the first weeks of life and neurodevelopmental outcome until the age of 5 years in very preterm infants. Pediatrics 2002;110:534–539.


Fig. 1
Steroid biosynthesis. The fetal zone of the human fetal adrenal cortex is capable of performing the reactions in the box (dotted line). DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone sulfate; 11βHSD, 11β hydroxysteroid dehydrogenase.
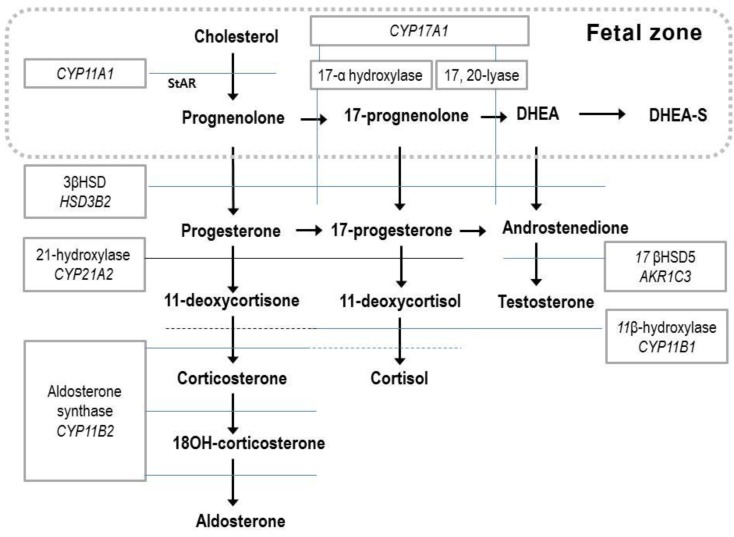
Fig. 2
Maternal-placental-fetal endocrine interaction. DHEA, dehydroepiandrosterone; E2, estradiol; E3, estrone; MDI3, monoamine deiodinase, type 3; T2, 3,5-diiodothyronine; T3, triiodothyronine; T4, thyroxine; rT3, reverse triiodothyronine; TRH, thyrotropin-releasing hormone; hCG, human chorionic gonadotropin; 11βHSD, 11β hydroxysteroid dehydrogenase; 17βHSD, 17β hydroxysteroid dehydrogenase; 3βHSD, 3β hydroxysteroid dehydrogenase.

Fig. 3
Approximate timeline of thyroid gland maturation in the human fetus. T4, thyroxine; T3, triiodothyronine; TSH, thyroid-stimulating hormone; TRH, thyrotropin-releasing hormone.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


