Transient intubation for surfactant administration in the treatment of respiratory distress syndrome in extremely premature infants
Article information
Abstract
Purpose
To investigate the effectiveness of transient intubation for surfactant administration and extubated to nasal continuous positive pressure (INSURE) for treatment of respiratory distress syndrome (RDS) and to identify the factors associated with INSURE failure in extremely premature infants.
Methods
Eighty-four infants with gestational age less than 28 weeks treated with surfactant administration for RDS for 8 years were included. Perinatal and neonatal characteristics were retrospectively reviewed, and major pulmonary outcomes such as duration of mechanical ventilation (MV) and bronchopulmonary dysplasia (BPD) plus death at 36-week postmenstrual age (PMA) were compared between INSURE (n=48) and prolonged MV groups (n=36). The factors associated with INSURE failure were determined.
Results
Duration of MV and the occurrence of BPD at 36-week PMA were significantly lower in INSURE group than in prolonged MV group (P<0.05), but BPD plus death at 36-week PMA was not significantly different between the 2 groups. In a multivariate analysis, a reduced duration of MV was only significantly associated with INSURE (P=0.001). During the study period, duration of MV significantly decreased over time with an increasing rate of INSURE application (P<0.05), and BPD plus death at 36-week PMA also tended to decrease over time. A low arterial-alveolar oxygen tension ratio (a/APO2 ratio) was a significant predictor for INSURE failure (P=0.001).
Conclusion
INSURE was the noninvasive ventilation strategy in the treatment of RDS to reduce MV duration in extremely premature infants with gestational age less than 28 weeks.
Introduction
Exogenous surfactant replacement for the treatment of respiratory distress syndrome (RDS) and improvement in mechanical ventilation (MV) therapy dramatically reduced the morbidity and mortality of preterm infants [1,2]. However, MV itself can induce lung injury and lead to the subsequent occurrence of bronchopulmonary dysplasia (BPD) [3,4]. Therefore, noninvasive ventilation strategies in the treatment of RDS have been widely recommended and used for many years [5-8]. Many studies have suggested that the early application of nasal continuous positive airway pressure (nCPAP) for RDS treatment is effective and can reduce the occurrence of lung injury [5-9]. Due to an expected synergistic effect, surfactant administration with nCPAP, has been regarded as an ideal non-invasive method for the treatment of RDS [9].
The transient intubation for surfactant administration and then extubated to nCPAP (INSURE) method has been widely applied as a less invasive method for RDS treatment, because it has been reported in several studies that INSURE can reduce the duration of MV and the incidence of BPD [5,10-13]. Dani et al. [12] have confirmed that INSURE decreased the duration of MV in infants less than 30-weeks’ gestation compared to infants managed with prolonged MV after surfactant administration. Similarly, Reininger et al. [13] reported that the INSURE method significantly reduced the need for MV in infants between 29 to 35-weeks gestation with mild-to-moderate RDS. However, the effectiveness of the INSURE method is still debatable due to inconsistent study results [14-16], and the lack of a standardized guideline particularly in extremely premature infants. The failure rate of INSURE has been reported from 9% to 40% [17-20].
Therefore, we intended to investigate the therapeutic effectiveness of the INSURE method for the treatment of RDS in extremely premature infants with a gestational age of less than 28 weeks, with the expectation that this procedure could reduce the duration of MV and the occurrence of BPD plus death at 36-week PMA, and examined the factors associated with INSURE failure.
Materials and methods
1. Subjects
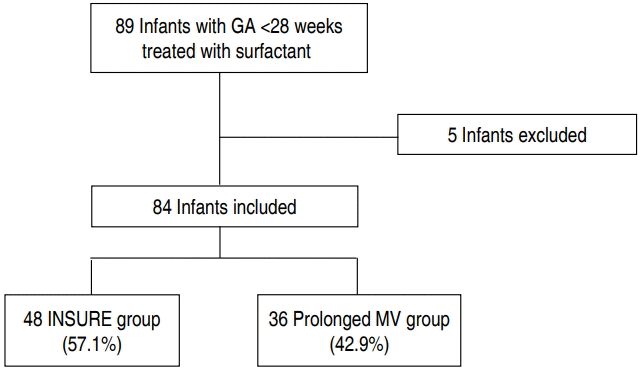
Eighty-nine extremely premature infants with a gestational age of less than 28 weeks who were treated with surfactant in the neonatal intensive care unit (NICU) of Dankook University Hospital between January 2008 and December 2015 were included. Five infants with congenital anomalies, congenital infection, early death due to severe asphyxia despite extensive resuscitation were excluded. Among the remaining 84 infants, 48 infants (57.1%) were extubated to nCPAP after the administration of surfactant (INSURE group), and 36 infants (42.9%) were treated with prolonged MV after the administration of surfactant (prolonged MV group) (Fig. 1). The perinatal and neonatal characteristics and short-term pulmonary outcomes during admission were retrospectively reviewed and compared between the 2 groups. This study was approved by the institutional review board of Dankook University Hospital (IRB No.2015-01-009-001).
2. Strategies of surfactant administration
We defined the INSURE method for RDS treatment as transient intubation for surfactant administration, then extubated to nCPAP within 1 hour, based on a previous study [16]. Therefore, infants who were able to be extubated to nCPAP within 1 hour after surfactant administration were classified as the INSURE group, and other infants who were treated with MV longer than 1 hour after surfactant administration were classified as the prolonged MV group. In our NICU, the INSURE method has been gradually applied for treatment of RDS since 2008. However, INSURE was applied only for infants with a gestational age over 28 weeks in the first 2 years (2008 to 2009). Thereafter, its application has gradually extended to extremely premature infants with a gestational age of less than 28 weeks. Therefore, the prolonged MV group included the premature infants with a gestational age of less than 28 weeks treated with prolonged MV after surfactant administration during the first 2 years since the application of INSURE. INSURE success was defined as no reintubation within 72 hours after extubation to nCPAP, and INSURE failure was defined as reintubation within 72 hours after extubation. Reintubation after INSURE was considered in infants who met one of the following criteria: FiO2 more than 0.4 with at least 30 minutes of nCPAP to maintain oxygen saturation between 85% to 92%, respiratory acidosis defined as pH<7.20 and PaCO2>65 mmHg on arterial or capillary blood gas, or frequent apnea (more than 4 episodes of apnea per hour or more than 2 episodes of apnea per hour when ventilation with bag and mask was required) [14]. In infants less than 28 weeks of gestation, prophylactic surfactant administration was preferentially considered at the place of delivery, and rescue administration of surfactant was performed in cases where prophylactic administration was not available. The rescue administration of surfactant was performed if the chest radiography showed a typical RDS pattern and infants needed FiO2 of 0.3 or more to maintain 80% oxygen saturation, and the administration of surfactants was done within 2 hours after birth in most infants. Two kinds of surfactant, Curosurf (Chiesi Pharmaceuticals, Parma, Italy) or Surfacten (Tokyo Tanabe Co., Japan) were randomly used without any preference criteria.
3. Pulmonary and neonatal outcomes
The duration of MV and BPD plus death at 36-week PMA were compared between the INSURE and prolonged MV groups as major pulmonary outcomes. Other variables related to pulmonary outcomes such as durations of nCPAP and oxygen supplement, oxygen dependency at 28 days old, BPD at 36-week PMA and postnatal steroid use for BPD were compared between the 2 groups. The changes in major pulmonary outcomes over time according to the changing rate of INSURE application during the study period were also evaluated.
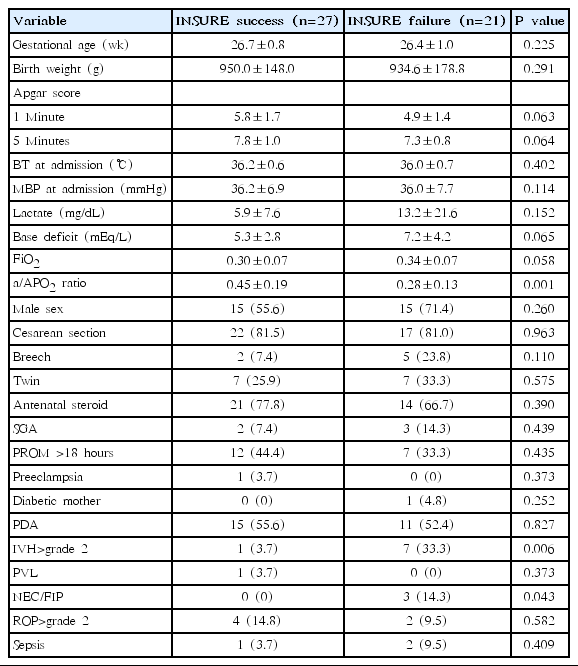
Gestational age, birth weight, sex, 1- and 5-minute Apgar scores, and other perinatal factors associated with pregnancy and delivery were evaluated. Clinical or laboratory findings of enrolled infants such as body temperature and mean blood pressure (MBP) at admission, initial lactate and base deficit were evaluated and compared between the INSURE and prolonged MV group. An initial or presurfactant arterial-alveolar oxygen tension ratio (a/APO2 ratio) for infant with prophylactic or rescue treatment of surfactant were evaluated. a/APO2 ratio (PaO2/PAO2) is known as an index of gas exchange function and the PAO2 was defined as follows; (PB–PH2O)×FiO2–PaCO2×[FiO2+(1–FiO2)/R] where PB is the barometric pressure (760 mmHg), PH2O is the patient’s water vapour pressure (47 mmHg), PaCO2 is the arterial CO2 tension (40 mmHg) and R is the gas exchange ratio (0.8) [21].
Additionally, neonatal outcomes such as patent ductus arteriosus (PDA), pulmonary hemorrhage, intraventricular hemorrhage (IVH >grade 2), periventricular leukomalacia, necrotizing of enterocolitis (NEC) or focal intestinal perforation (FIP), retinopathy of prematurity (>grade 2) and culture proven sepsis were compared between the INSURE and prolonged MV groups. BPD was defined as the need for any supplemental oxygen at 36-week PMA, in which mild BPD was excluded according to BPD classification [22].
4. Predictors for INSURE failure
To evaluate the risk factors to predict INSURE failure, the above clinical and laboratory factors presumed to be related with INSURE failure were compared between the INSURE success and failure group.
5. Statistical analysis
Data were analyzed using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA). Chi-square test, Fisher exact test, Student t test, Mann-Whitney U test, and analysis of variance were used for analyses. P values less than 0.05 were considered significant. To determine whether INSURE might independently affect major pulmonary outcomes, multiple linear regression and logistic regression were performed only on the significant variables in the univariate analysis. The risk factors associated with INSURE failure were analyzed.
Results
1. Clinical characteristics
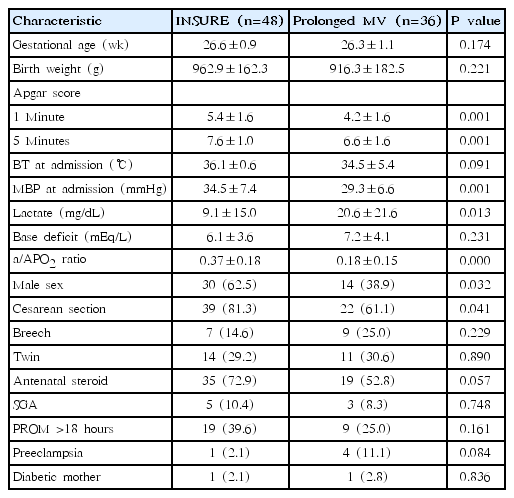
Mean gestational ages and mean birth weight were not significantly different between the INSURE and prolonged MV groups (26.6±0.9 weeks vs. 26.3±1.1 weeks and 962.9±162.3 g vs. 916.3±182.5 g, respectively, P>0.05). One- and 5-minute Apgar scores were significantly lower in the prolonged MV group than in the INSURE group (4.2±1.6 vs. 5.4±1.6 and 6.6±1.6 vs. 7.6±1.0, respectively, P< 0.05). The MBP at admission was significantly lower and lactate level was higher in the prolonged MV group than in the INSURE group (P<0.05). The mean a/APO2 ratio in the INSURE group was significantly higher than in the prolonged MV group (0.37±0.18 vs. 0.18±0.15, P=0.000). Male sex was dominant in the INSURE group, and more cesarean section were done in this group. There was a tendency of more antenatal steroid use in the INSURE group (Table 1).
2. Pulmonary and neonatal outcomes
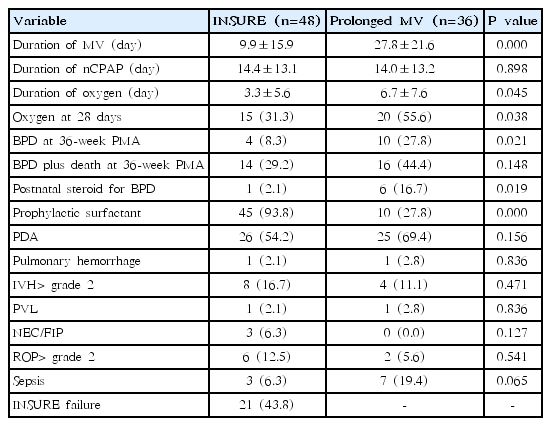
The mean duration of MV was significantly shorter in the INSURE group than in the prolonged MV group (9.9±15.9 days vs. 27.8± 21.6 days, P=0.000), and the rate of BPD at 36-week PMA was significantly lower in the INSURE group than in the prolonged MV group (8.3% vs. 27.8%, P=0.021). However, the rate of BPD plus death at 36-week PMA was not significantly different between the two groups (29.2% vs. 44.4%, P=0.148). Additionally, infants treated with INSURE had a shorter duration of oxygen supplement (P=0.045), and were less dependent on oxygen at 28 days after birth (P=0.038). The postnatal use of steroids for BPD was more common in the prolonged MV group (P=0.019) and surfactants were more commonly administered prophylactically in the INSURE group (P=0.000). Other neonatal complications were not significantly different between the 2 groups. The rate of INSURE failure was 43.8% (Table 2).
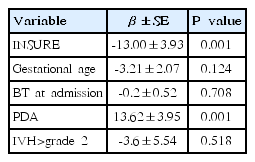
Multivariate analysis for duration of MV was performed only on the significant variables in the univariate analysis. In a multiple regression analysis, INSURE was only significantly associated with the decreasing duration of MV (β±standard error [SE], -13.00±3.93, P=0.001) (Table 3) but not associated with the occurrences of BPD and BPD plus death at 36-week PMA. PDA was also significantly associated with the increased duration of MV (β±SE, 13.62±3.95, P=0.001) (Table 3).
During the study period, the duration of MV decreased significantly with the increasing rate of INSURE application (P< 0.05). The rate of BPD plus death at 36-week PMA also tended to decrease over time (Fig. 2).
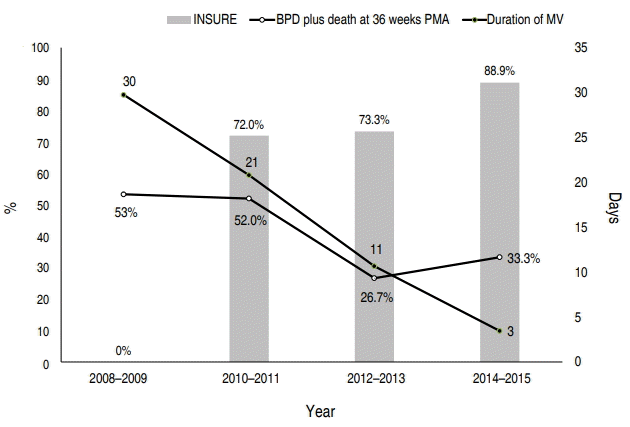
Changes in major pulmonary outcomes over time during the study period. The duration of mechanical ventilation (MV) decreased significantly with the increasing rate of INSURE application (P<0.05). The rate of BPD plus death at 36-week postmenstrual age (PMA) also tended to decrease over time. INSURE, intubation, surfactant administration, and extubated to nCPAP; nCPAP, nasal continuous positive pressure ventilation; BPD, bronchopulmonary dysplasia.
3. Risk factors for INSURE failure
The a/APO2 ratio was a significant risk factor for INSURE failure (P=0.000). IVH>grade 2 and NEC/FIP were also associated with INSURE failure (Table 4). The area under the curve (AUC) of receiver operating characteristic (ROC) curve for the a/APO2 ratio was 0.80 (95% CI, 0.68–0.92; P=0.000) and the cutoff value of the a/APO2 ratio for INSURE failure prediction was estimated at 0.31 with 70.4% sensitivity and 71.4% specificity (Fig. 3).
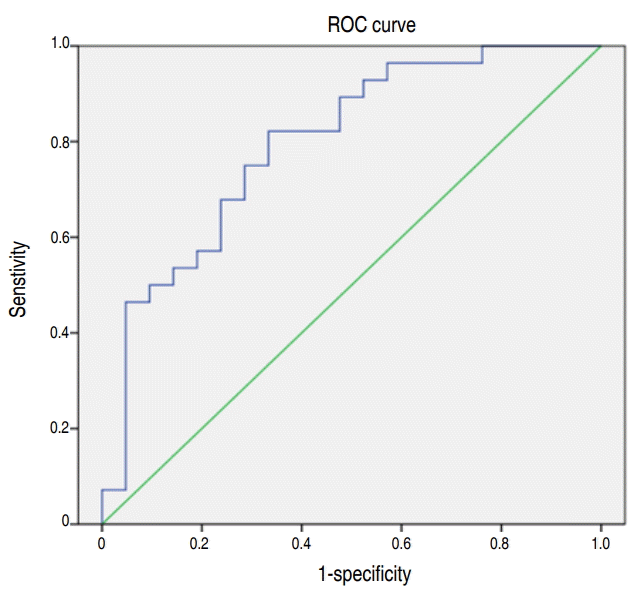
Receiver operating characteristic (ROC) curve for the arterialalveolar oxygen tension ratio (a/APO2 ratio) for INSURE success prediction. The area under the curve (AUC) was 0.80 (95% confidence interval, 0.68–0.92; P=0.000). The cutoff value of the a/APO2 ratio for INSURE success prediction was estimated at 0.31 with 70.4% sensitivity and 71.4% specificity. INSURE, intubation, surfactant administration, and extubated to nCPAP; nCPAP, nasal continuous positive pressure ventilation; a/APO2 ratio, arterial-alveolar oxygen tension ratio.
Discussion
Noninvasive ventilation is now preferentially recommended in various studies to treat preterm neonate with RDS to reduce lung injury. Even for extremely premature infants, nCPAP is recommended as an alternative to intubation in patients who meet specific criteria [23-25]. In a recent review from European consensus guidelines, it is strongly recommended that nCPAP be initiated from the beginning of treatment in all infants with RDS, including even those with less than 30 weeks of gestation who do not need intubation. This review also mentioned that the INSURE technique can be applied to infants who are failing on nCPAP [5].
Several previous studies have reported the validity of the application of the INSURE method for the treatment of RDS in premature infants [10-13,26-28]. In a study including 279 premature infants born at 27- to 31+6-week gestation with RDS, Rojas et al. [27] reported that the need for MV was significantly reduced in the INSURE group compared to the nCPAP group (26% vs. 39%, P<0.05), and less air-leak and less chronic lung disease was observed in the INSURE group. In another study that included 125 infants with RDS and a gestational age less than 30 weeks, INSURE was effective in 91% of infants who required a FiO2>30% without need for MV during the first hour of life [28].
Similar to the results of previous studies, this study showed that the application of INSURE method for treatment of RDS in extremely premature infants with a gestational age less than 28 weeks reduced the duration of MV and the occurrence of BPD at 36-week PMA (P<0.05) but could not reduce the occurrence of BPD plus death at 36-week PMA. Additionally, the duration of oxygen supplement, dependency of oxygen at 28 days and use of a postnatal steroid were significantly decreased in the INSURE group. However, in a multivariate analysis, the reduced duration of MV was only significantly associated with INSURE (P=0.001), but the occurrences of BPD or BPD plus death at 36-week PMA were not significantly associated with INSURE. PDA was also a significant independent factor for an increase in the duration of MV (P=0.001), presumably because PDA could deteriorate lung function.
The cause of reintubation after INSURE is known to be primarily related to the severity of RDS. Cherif at al. [18] reported that the rate of INSURE failure was 32.1% in a study of 109 neonates with 27– 34 weeks of gestation, and the arterial partial pressure of carbon dioxide, mean a/APO2 ratio and severity of radiological grade for RDS were significantly associated with INSURE failure. Another study reported that the rate of reintubation after INSURE application for RDS was 30% in newborn infants with a gestational age less than 30 weeks and a birth weight less than 750 g, and RDS severity could affect the success rate of INSURE in a multivariate analysis [19]. In a recent study, Najafian et al.20) suggested that birth weight was the only predicting factor for the INUSURE success. In this study, INSURE failure rate was 43.8%, which was higher than the results of other studies, presumably because this study mainly included extremely premature infants less than 28 weeks of gestation. An initial or presurfactant low a/APO2 ratio was a significant risk factor for INSURE failure, which suggested that INSURE was more likely to fail if gas exchange in the alveoli was severely impaired due to severe RDS. IVH>grade 2 and NEC/FIP were also significant factors associated with INSURE failure. But, because their numbers were few and they were thought to mainly reflect the progression after application of INSURE, these factors were not considered as main risk factors associated with INSURE failure. The estimated cutoff value of presurfactant a/APO2 ratio in the ROC curve was 0.31, which might be a predictive marker for the INSURE failure.
In this study, INSURE was not used for extremely premature infants with a gestational age of less than 28 weeks during 2008–2009, but there has been a gradual increase in the rate of extremely premature infants treated by INSURE from 2010 to 2015. During this period, the duration of MV was markedly reduced, and the rates of BPD plus death at 36-week PMA was tended to decrease over time. However, this tendency of decrement in BPD plus death at 36-week PMA could not be completely explained by INSURE, because the pathogenesis of BPD is multifactorial and the improvement of the other risk factors associated with the development of BPD during the study period was also thought to be related.
In this study, infants in prolonged MV group had lower 1- and 5-minute Apgar scores, lower MBP, a tendency of less antenatal steroid, a lower a/APO2 ratio, and a higher level of lactate than infants in the INSURE group, which suggested the possibility that INSURE might be primarily applied to infants with less severe RDS or less severe clinical conditions immediately after birth, and more severe cases might be included in the prolonged MV group. Thus, this baseline discrepancy of the clinical and laboratory characteristics between the 2 groups could affect the differences in pulmonary outcomes between the INSURE and prolonged MV groups. Nevertheless, INSURE in this study was significantly associated with the reduced duration of MV (in a multivariate analysis after adjustment for significant variables in a univariate analysis). In addition, the absence of INSURE use in extremely premature infants in the first 2 years of the study period and a lack of clear guidelines for patient selection and the application of INSURE might affect the results.
There are many ongoing studies to find the best strategy of surfactant administration for the treatment of RDS in preterm neonates. Minimally invasive surfactant therapy (MIST) and similar approaches, called less invasive surfactant administration, are studied as alternative ways to reduce the duration of MV and the incidence of BPD [29-31]. Compared directly with the INSURE group, MIST has shown more promising pulmonary outcomes in one study [32], however, there is still controversy surrounding the effectiveness [33].
In conclusion, this study suggested that INSURE application for the treatment of RDS in extremely premature infants with gestational age less than 28 weeks might reduce the duration of MV but not the occurrence of BPD plus death at 36-week PMA. An initial or presurfactant low a/APO2 ratio was a significant risk factor for INSURE failure, which suggested that INSURE was more likely to fail in infants if gas exchange in the alveoli was severely impaired due to severe RDS. A prospective randomized study with more patients is needed to determine the potential benefits of the INSURE strategy for the treatment of RDS and to establish evidence-based guidelines for the INSURE method.
Notes
No potential conflict of interest relevant to this article were reported.