Introduction
Osteosarcoma is the most common primary malignant tumor of the bone1,2). In Korea, approximately 50 children and adolescents are newly diagnosed as having osteosarcoma per year3). Advances in combination chemotherapy and surgical technique have greatly improved the survival of these patients1,2). Currently, more than 60% of patients with localized disease are cured1,2). In this article, the clinical characteristics and treatment outcome of Korean children and adolescents will be reviewed.
Current status of Korean children and adolescents with osteosarcoma: results of the Korean Society of Pediatric Hematology and Oncology study
In 2009, the Korean Society of Pediatric Hematology and Oncology conducted a retrospective study on the outcome of Korean children and adolescents with osteosarcoma treated between 1989 and 20094). Data on a total of 320 patients were collected from 19 institutions. There were 192 male and 128 female patients with a median age of 11.8 years (range, 3.3-35.7 years) at the time of diagnosis. The distal femur (52.3%) was the most frequently affected site, followed by the proximal tibia (19.5%) and proximal humerus (11.1%). The most common histological subtype was osteoblastic, and 64 patients (19.8%) had distant metastasis at the time of diagnosis. Osteosarcoma developed as a secondary malignancy in three cases.
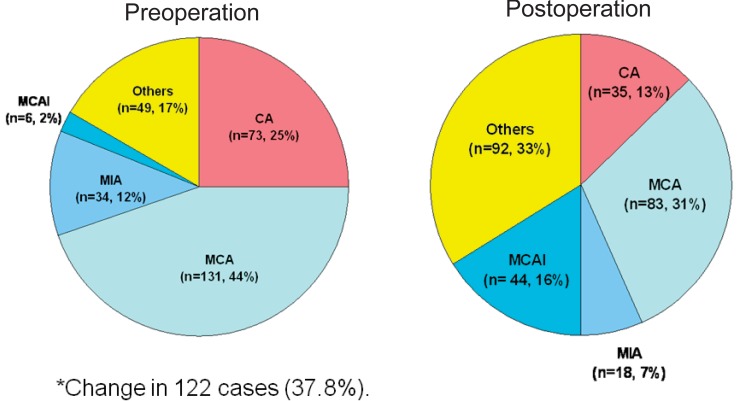
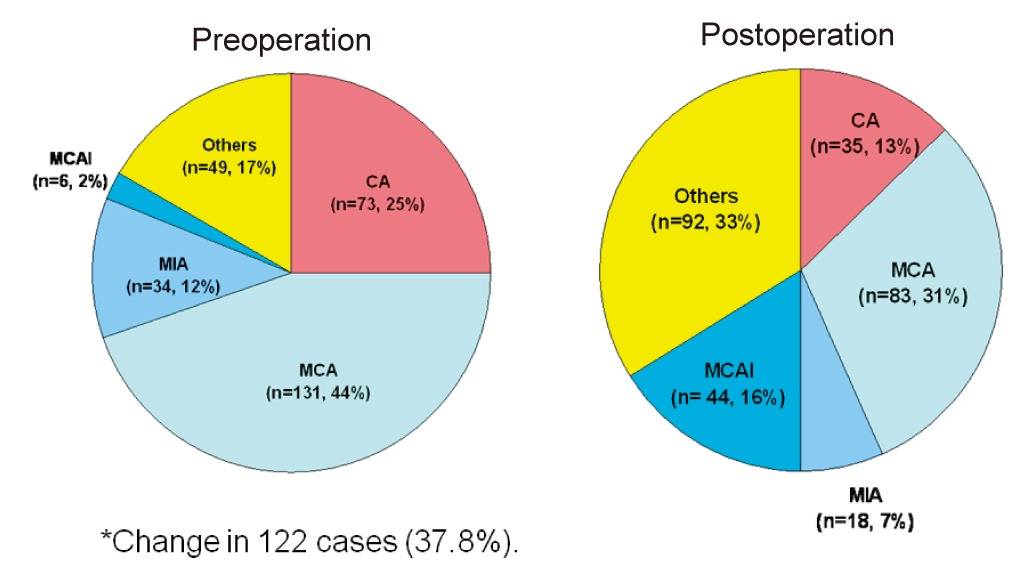
Treatments were heterogeneous, depending on treatment era or institutional policy. The majority of patients (252 cases, 78.0%) were treated in a standard fashion with preoperative chemotherapy followed by surgery and postoperative chemotherapy. Of the 280 patients that underwent surgery, limb salvage was performed in 253 cases (90.4%). High-dose methotrexate (HD MTX), cisplatin, doxorubicin, and ifosfamide were the core components of combination chemotherapy. Chemotherapy regimens differed between institutions (Fig. 1). Postoperative chemotherapy was modified in 122 cases (37.8%) due to poor histological response (viable tumor cell>10%) to preoperative chemotherapy.
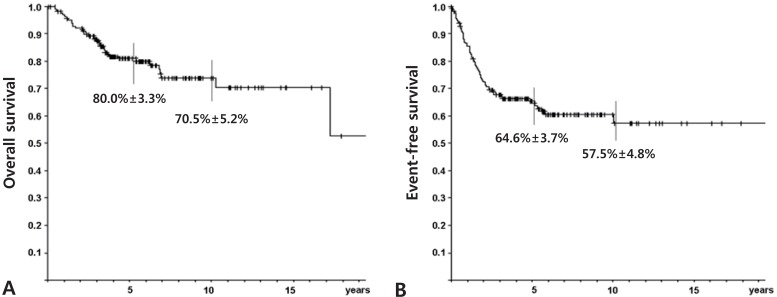
For survival analysis, data from the 225 cases followed-up for more than 2 years were evaluated using the Kaplan-Meier method. The probability of overall (OS) and event-free survival (EFS) at 5 years were 70.9% and 60.7%, respectively. 70.9% and 60.7%±3.6%, respectively. The 5-year OS and EFS rates were better for the 184 patients who presented without metastasis at the time of diagnosis, 80.0% and 64.6%, respectively (Fig. 2). The association between clinicopathological variables and survival was evaluated. We found that the presence of metastasis at the time of diagnosis and histological response to preoperative chemotherapy influenced survival (Table 1).
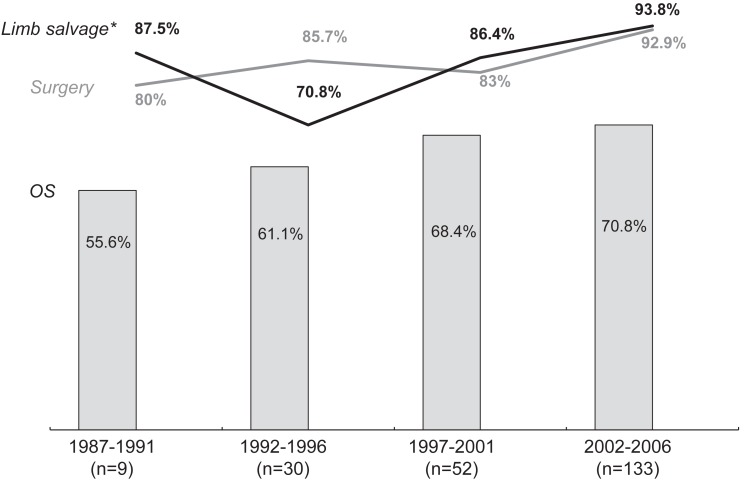
Fig. 3 shows the improvements made in osteosarcoma treatment over the past two decades. Currently, the treatment outcomes of Korean children and adolescents are comparable to those of American or European patients.
Challenges
1. Recurrent or refractory tumors
Despite improvements with a multidisciplinary treatment approach, 30%-40% of osteosarcoma patients still relapse and eventually succumb to the disease1,5). Prognosis of patients with recurrent or refractory osteosarcoma is poor1,6,7,8). Lee et al.7) reported that the 5-year survival rate of 180 patients with recurrent osteosarcoma was 13%. The survival rate was influenced by the site of recurrence (lung, 39%; local, 0%; lung and bone, 25%; others, 12%; P<0.05), recurrence-free interval (<12 months, 13%; ≥12 months, 44%, P<0.05), and treatment modality after recurrence (with surgery, 38%; without surgery, 11%; P<0.05). Currently, standard guidelines do not exist for the treatment of these patients6,7,8). Generally, local control surgery is performed whenever possible and additional adjuvant chemotherapy is given. Combination chemotherapy comprising ifosfamide, carboplatin, and etoposide is the most frequently used regimen6,7,8). Patients who experience treatment failure with this combination proceed to receive gemcitabine and docetaxel (GEMDOC) chemotherapy8).
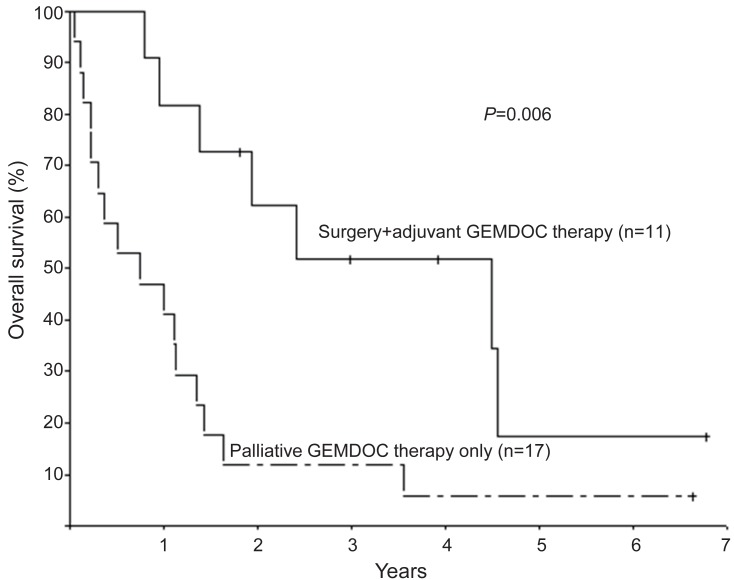
The efficacy of GEMDOC has been reported in various sarcomas, including Ewing sarcoma, malignant fibrous histiocytoma, synovial sarcoma, angiosarcoma and osteosarcoma9,10,11,12,13,14,15,16). Prompted by encouraging results from adult studies, GEMDOC chemotherapy is used in children and adolescents with recurrent or refractory bone sarcomas13,14,15,16). Due to the rarity of bone sarcomas, case series reporting the efficacy of GEMDOC chemotherapy analyzed patients with various pathologic diagnoses13,14,15,16). Data from the Korea Cancer Center Hospital8) showed that GEMDOC chemotherapy had some activity in osteosarcoma, and better than expected survival after GEMDOC chemotherapy was observed in patients both with and without surgery. The 28 patients (aged 5.0-19.7 years) received a total of 96 courses of chemotherapy (median, 3 courses; range, 1-8 courses) and were followed-up for a median of 14.9 months (range, 0.6-81.4 months). Eleven patients received GEMDOC chemotherapy after surgery as adjuvant chemotherapy. Seventeen patients received GEMDOC chemotherapy as palliative therapy and were eligible for response evaluation. Of these patients, 3 (17.6%) experienced a complete response (CR, including 2 metabolic CR); 1 (5.9%), a partial response (PR), and 3 (29.4%), stable disease (SD). The objective response rate (CR+PR) and tumor control rate (CR+PR+SD) were 23.5% and 41.2%, respectively. The median duration of response was 11.2 months (range, 2.8-14.6 months). OS at 1 year was 53.6%±9.4% and patients who received GEMDOC chemotherapy as adjuvant chemotherapy fared better than those who received GEMDOC chemotherapy as palliative therapy (Fig. 4).
2. Local therapy for recurrent, refractory, or unresectable tumors
Effective local control is a crucial element in curing osteosarcoma and complete surgery is the most effective local control measure1,2,5). The extremities are the most frequently affected sites of osteosarcoma1,2), and complete surgery is feasible in the majority of cases. However, cases with unresectable lesions or cases where surgery will not yield an acceptable functional outcome require other local control measures. Radiotherapy has been a mainstay of nonsurgical local tumor control and plays a major role in the treatment of various sarcomas, such as Ewing sarcoma or rhabdomyosarcoma17). Osteosarcoma has a low radiosensitivity and few reports have described effective local control using radiotherapy18,19,20).
New treatment approaches utilizing both radiotherapy and chemotherapy are underway for various malignancies21). Chemotherapeutic agents are administered concurrently or sequentially with radiotherapy and exert both systemic (anticancer) and local (radiosensitizing) effects21,22). The presumed mechanisms by which chemotherapeutic agents interact with radiotherapy are as follows21,22): (1) inhibition of radiation damage repair, (2) perturbation of cell cycling to increase the fraction of G2/M-phase cells, and (3) specific action on hypoxic cell populations. Cisplatin, 5-fluorouracil (5-FU), ifosfamide, gemcitabine, and docetaxel are frequently used in chemoradiotherapy21). The choice, optimal combination, and scheduling of chemotherapy with radiotherapy remain in evolution. For patients with unresectable recurrent or refractory osteosarcoma, a novel or third-line chemotherapeutic agent might be preferred. Sequential or concurrent chemoradiotherapy using gemcitabine showed efficacy in the treatment of nonsmall cell lung cancer23). GEMDOC combined with samarium-153 ethylene diamine tetramethylene phosphonate showed promising results in osteosarcoma patients24,25). Still, there are no data on the treatment outcome of GEMDOC combined with external beam radiotherapy.
We analyzed the outcomes of eight patients with unresectable recurrent or refractory osteosarcoma who were treated with radiotherapy and GEMDOC chemotherapy at the Korea Cancer Center Hospital26). The tumor sites were the bone in six patients and the lung in two patients. Patients received a median of 3.5 courses of GEMDOC chemotherapy (range, 2-6 courses) and the median dose of radiotherapy was 50.0 Gy (range, 46-84 Gy). The objective response rate was 50.0% (2 complete metabolic responses, 1 partial response, and 1 partial metabolic response) (Fig. 5). Responses were maintained for 4.6, 6.1, 6.2, and 13.7 months, respectively. Patients were followed for a median of 8.1 months (range, 2.7-84.6 months) and the median progression-free survival after treatment was 3.6 months (range, 1.1-13.7 months). At the time of writing, two patients were alive, one was lost to follow-up, and five are deceased.
New or other chemotherapeutic agents?
No other agent has come close to replacing the four standard chemotherapeutic agents: HD MTX, cisplatin, doxorubicin, and ifosfamide. Etoposide has shown limited activity when administered as a single agent27) but may enhance the effect of other drugs such as cyclophosphamide28,29). When administered as single agents, 5-azacytidine30), 5-FU/leucovorin31), iproplatin32,33), ecteinascidin-74334), and topotecan35) seem to be more or less inactive.
In the few reported studies, the benefits of high-dose chemotherapy and autologous peripheral blood stem cell rescue are still unproven for patients with osteosarcoma36,37).
The American intergroup trial INT0133 evaluated the efficacy of liposomal muramyl-tripeptide-ethanolamine (MTP), an immune modulator38,39). A recent analysis reported a trend for better EFS (P=0.08) and improved OS (P=0.03) for the liposomal MTP arm39). Liposomal MTP is available in Korea via the Orphan Drug Center. However, routine use is hampered due to its tremendous cost and the lack of confirmatory clinical data.
Inhibitors of IGF1R and its downstream pathways have shown promise in preclinical models of osteosarcoma40). Tumor responses to anti-IGF1R therapy have been published for a total of 7 patients with osteosarcoma41). The outcomes of additional phase I and phase II studies have yet to be reported. The Italian Sarcoma Group reported that a combination of sorafenib and the mammalian target of rapamycin inhibitor everolimus showed activity as a further-line treatment for patients with advanced or unresectable osteosarcoma42).
Conclusions
The survival of Korean patients with osteosarcoma has improved over the last 20 years and is comparable to that reported in major Euro-American studies. However, patients with metastasis at the time of diagnosis and those with relapsed tumors have poor prognosis. Currently, the search for new chemotherapeutic agents and alternative treatment strategies, such as therapies targeting biological markers, angiogenesis, and the immune system, is underway to improve the survival of these patients.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


