Combined chemotherapy and intra-arterial chemotherapy of retinoblastoma
Article information
Abstract
Purpose
Retinoblastoma (RB) is the most common primary malignant intraocular tumor in children. Although systemic chemotherapy has been the primary treatment, intra-arterial chemotherapy (IAC) represents a new treatment option. Here, we performed alternate systemic chemotherapy and IAC and retrospectively reviewed the efficacy and safety of this approach.
Methods
Patients diagnosed with intraocular RB between January 2000 and December 2011 at Severance Children's Hospital, Yonsei University, were reviewed. Before February 2010, the primary treatment for RB was chemotherapy (non-IAC/CTX). Since February 2010, the primary treatment for RB has been IAC (IAC/CTX). External beam radiotherapy or high-dose chemotherapy were used as "last resort" treatments just prior to enucleation at the time of progression or recurrence during primary treatment. Enucleation-free survival (EFS) and progression-free survival were assessed.
Results
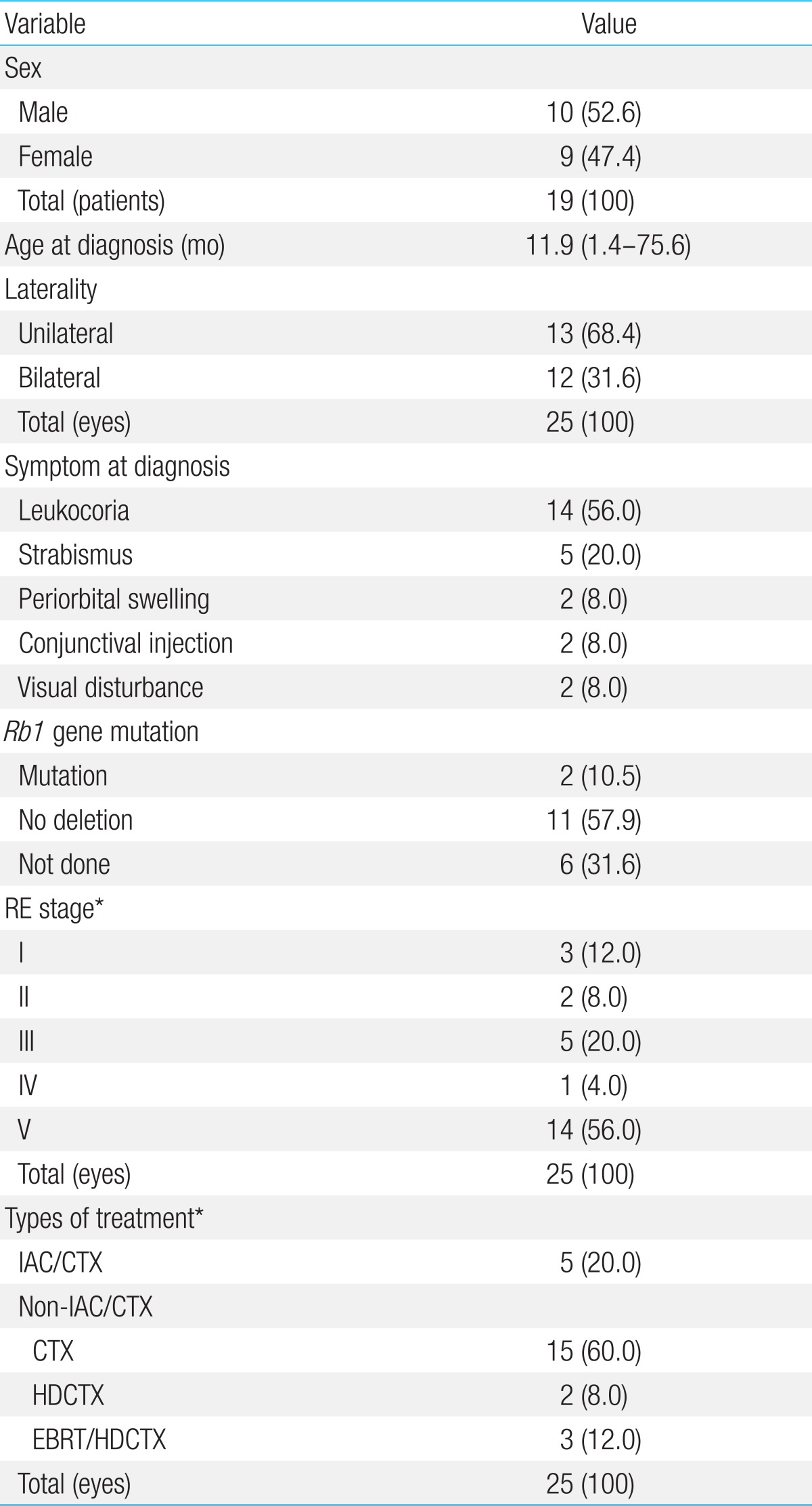
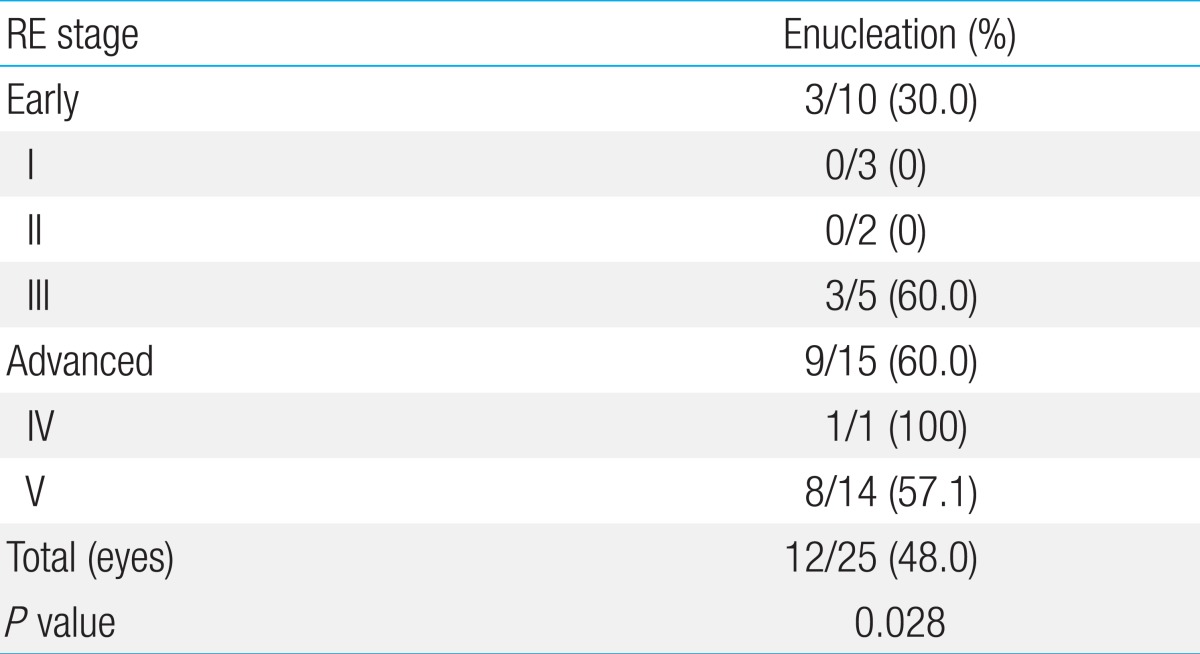
We examined 19 patients (median age, 11.9 months; range, 1.4 to 75.6 months) with a sum of 25 eyes, of which, 60.0% were at advanced Reese Ellsworth (RE) stages. The enucleation rate was 33.3% at early RE stages and 81.8% at advanced RE stages (P=0.028). At 36 months, EFS was significantly higher in the IAC/CTX group than in the non-IAC/CTX group (100% vs. 40.0%, P=0.016). All 5 patients treated with IAC achieved eye preservation, although most patients were at advanced RE stages (IV-V).
Conclusion
Despite the limitation of a small sample size, our work shows that an alternative combined approach using IAC and CTX may be safe and effective for eye preservation in advanced RB.
Introduction
Retinoblastoma (RB) is known as one of the common primary malignant intraocular tumor of childhood which occurs in 1% of all tumors in infancy, 12 out of 2 million children under 4 years old and the median age is under 12 months old. There are two main forms of RB; genetic and somatic. Patients with the germline mutation of Rb1 gene (genetic form) have significant risk of secondary malignancy increased with radiation treatment for the primary cancer1).
External beam radiotherapy (EBRT) or enucleation was considered to be the only definite treatment in the past. The modalities achieve high survival rate, but also have significant limitations. Enucleation is performed in high stage unilateral RB (vitreous seeding), more progressed eye in bilateral eyes, vitreous hemorrhage, and complicated cataract. It is also performed as the final treatment of RB in the failure of other treatment modalities. EBRT can cause serious complications such as cataract, facial growth disturbance, optic neuropathy, and secondary malignancy. To improve survival, prevent serious complications and preserve eye sight, many other treatment modalities than enucleation and EBRT have been tried. Systemic control is necessary with chemotherapy especially in high risk RB2). The role is to increase long-term survival, reduce complications from EBRT, and avoid enucleation. Focal therapies such as laser photocoagulation, thermotherapy, and cryotherapy were effective in small tumors posterior to the equator of the eye1,3). The application of systemic chemotherapy and focal therapy greatly increased the survival rate and reduce complications.
Intra-arterial chemotherapy (IAC) with melphalan or other chemotherapeutic agents was lately introduced in the treatment of RB. IAC had been tried as the second line treatment after the failure of chemotherapy and focal treatment. Recently, IAC has been started to be used as first line treatment. IAC was effective for Reese Ellsworth (RE) stage IV to V, in which chemotherapy had not been effective. IAC had more than 70% of ocular salvage rates as a primary treatment. Success rates are inferior when it is used as the second line treatment, that is, after the failure of systemic chemotherapy or radiotherapy4,5).
In this study, the primary treatment of RB was combined chemotherapy with supplemental focal therapy, or IAC. When only IAC is applied as a sole primary treatment, it has some risks of recurrence or metastasis because pathologic risk factors cannot be assessed. In order to determine the need for adjuvant chemotherapy after enucleation, pathologic examination of enucleated specimen is necessary for exact risk assessment and need of adjuvant chemotherapy. High risk features include anterior chamber seeding, and choroidal involvement for metastasis. We added systemic adjuvant chemotherapy to IAC to prevent metastasis and recurrence6). We hereby analyzed retrospectively the efficacy and safety of the combined alternate IAC and chemotherapy approach.
Materials and methods
1. Patients
Total of 35 patients with intraocular RB who were evaluated and treated between January 2000 and December 2011 at Severance Children's Hospital, Yonsei University Health System were identified in this study. Among 35 patients, 16 were excluded due to insufficient information and follow up loss. Data was collected retrospectively for the remaining 19 patients with total of 25 eyes and numbered each eyes. There were 6 bilateral RB patients and each eye was numbered from 11 through 22. Age at diagnosis, gender, initial symptom at diagnosis, laterality, RE stage, and type of treatments, were assessed.
2. Stage
RE stage is the classification of intraocular RB to show prognostic significance for maintenance of sight when enucleation and EBRT were primary treatment options7-12). Ophthalmologists examined the tumor status, RE stage, and assessed the need for focal therapy.
3. Treatment
We divided the patients to 2 main groups, IAC/CTX group and non-IAC/CTX group. Non-IAC/CTX group include following subcategories; chemotherapy (CTX group), chemotherapy followed by high-dose chemotherapy (HDCTX group), and chemotherapy followed by EBRT and HDCTX (EBRT/HDCTX group). All RB patients without metastasis received chemotherapy or IAC as the primary treatment in order to avoid enculeation. Before 2010, the primary treatment for all RB was CTX. Since 2010, primary treatment for all RB has been IAC. EBRT or HDCTX was the last resort, just prior to enucleation at the time of progression or recurrence during primary treatments. In bilateral RB, we performed primary treatment without enucleation of more aggressive eye at the diagnosis.
1) Systemic chemotherapy and focal therapy
Systemic chemotherapy was performed to all patients and to all stages of RB. Patients received chemotherapy with or without focal therapies such as laser photocoagulation, transpupillary thermotherapy, and cryotherapy. Decision of need for focal therapy was determined by ophthalmologists through detailed examination under general anesthesia. Chemotherapy regimen consisted of carboplatin (D1 and D2, 200 mg/m2 or 6.7 mg/kg), vincristine (D1, 1.5 mg/m2 or 0.05 mg/kg), etoposide (D1 and D2, 150 mg/m2 or 5 mg/kg), and cyclosporin (D1 and D2, 360 mg/m2 or 12 mg/kg) in 3 weeks. Weight based dosage calculation were applied to the patients with weight less than 10 kg or age less than 36 months old.
2) EBRT and HDCTX
EBRT was mainly performed in patients diagnosed before year 2008. EBRT or HDCTX with peripheral blood stem cell transplantation was applied at the time of progression or recurrence after the primary treatment in order to preserve eye globes.
3) IAC
Since year 2010, IAC was applied as first line therapy performing alternately with chemotherapy to all patients who were diagnosed as RB without metastasis (IAC/CTX approach). Interval between IAC and chemotherapy was 3 weeks and we performed maximal 6 cycles of IAC or chemotherapy for each. IAC was performed by skilled radiologist specialized in intervention procedure. Femoral arterial puncture and insertion of Hunter catheter passing through aorta, common carotid artery, internal carotid artery, and finally Marathon microcatheter positioned at the ostium of ophthalmic artery. Melphalan (3-7.5 mg/kg/injection) was infused directly into ophthalmic artery for 30 minutes.
4) Enucleation
Enucleation was performed as the final treatment in high stage RB at the time of progression during the primary treatment or recurrence with no expected chance of preservation of eye sight.
5) Adverse effects
Adverse events were assessed by Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0.
4. Statistical analysis
Enucleation rate (ER) was defined as the proportion of enucleation according to the treatment groups and RE stage. Enucleation-free survival (EFS) was defined as the duration (months) from diagnosis to the enucleation. EFS was assessed at 60 months of follow-up for RE stage and 36 months for the treatment modalities because of the shorter median follow-up duration of the IAC/CTX group. Progression-free survival (PFS) was from the diagnosis to the event of receiving EBRT, HDCTX, and enucleation, whichever comes first. Statistical analysis was performed by using PASW ver. 18.0 (IBM Co., Armonk, NY, USA). Chi-square analysis was used for comparison of discontinuous variables, Mann-Whitney U test for nonparametic significance test, and Kaplan-Meier survival analysis for EFS and PFS. P value of <0.05 was considered statistically significant.
Results
1. Demographic characteristics
Median age at the time of diagnosis was 11.9 months old (range, 1.4 to 75.6 months). Six patients were diagnosed with bilateral RB (31.6%) and 13 were unilateral. Total eye number was 25. Leukocoria was the most common feature presented at the time of diagnosis (14/25, 56.0%), strabismus (5/25, 20.0%) was the second. According to RE stage, 60.0% of total eyes (15/25) were in advanced stage (IV and V). All patients survived with median follow up duration of 47.3 months (range, 12.2 to 134.4 months) (Table 1).
2. Treatment results
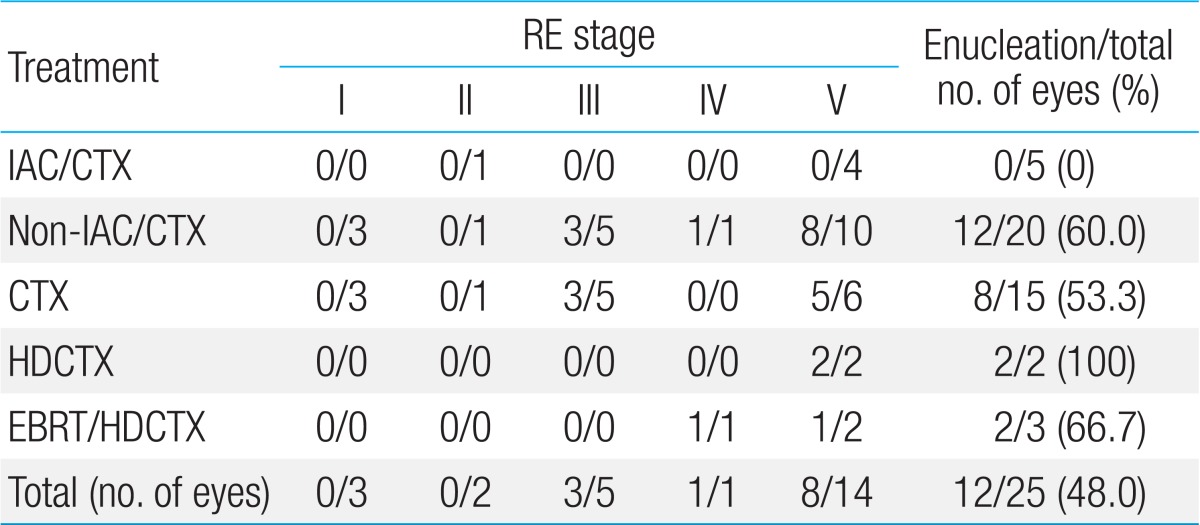
Distribution of patients according to the type of treatment modalities are as the following; IAC/CTX group was 5 eyes (20.0%), in non-IAC/CTX group was 20 eyes (80.0%). In non-IAC/CTX group, CTX group was 15 eyes (60.0%) with median of 10 cycles (3 to 20), HDCTX group was 2 eyes (8.0%), and EBRT/HDCTX group was 3 eyes (12.0%), respectively. ER according to the RE stage of RB was 30.0% (3/10) in early stage (I to III) with median eye survival time of 14.1 months (range, 1.0 to 94.3 months) and 60.0% (9/15) in advanced stage (IV to V) with median 31.3 months (range, 0.6 to 24.6 months) (Table 2). In all 25 eyes, ER was 48.0% (12/25). No eyes were enucleated in IAC/CTX group (0/5) whereas 60.0% (12/20) of eyes in non-IAC/CTX group were enucleated (P=0.016). Among enucleated eyes in non-IAC/CTX group, 53.3% (8/15) was CTX group and 100.0% (2/2) in HDCTX group, and 66.7% (2/3) in EBRT/HDCTX group (P=0.033) (Table 3). In non-IAC/CTX group, ER according to the RE stage of RB was 33.3% (3/9) in early stage (I to III) and 81.8% (9/11) in advanced stage (IV to V) (P=0.028). HDCTX and EBRT/HDCTX were all performed in patients with advanced RE stage (IV to V).
3. Eye preservation rate of IAC/CTX and its adverse events
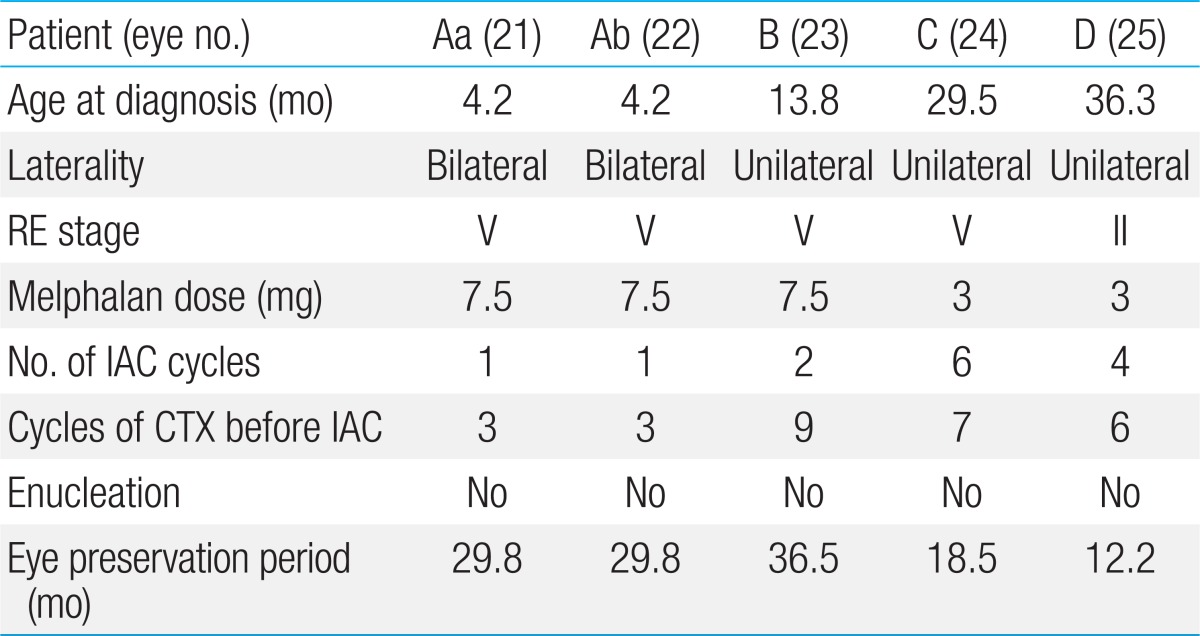
Four patients with total of 5 eyes received IAC (Table 4). Four out of 5 were in stage V and one was stage II. Melphalan dosage was 3 to 7.5 mg and IAC was delivered with median of 1 cycle (range, 1 to 5) and CTX was delivered with median of 6 cycles (range, 3 to 9 cycles) in IAC/CTX group. All eyes were preserved with median of 24.2 months of follow-up (range, 12.2 to 36.5 months). ER in IAC/CTX group was significantly lower than non-IAC/CTX groups (0% [0/5] vs. 60.0% [12/20], P=0.016).
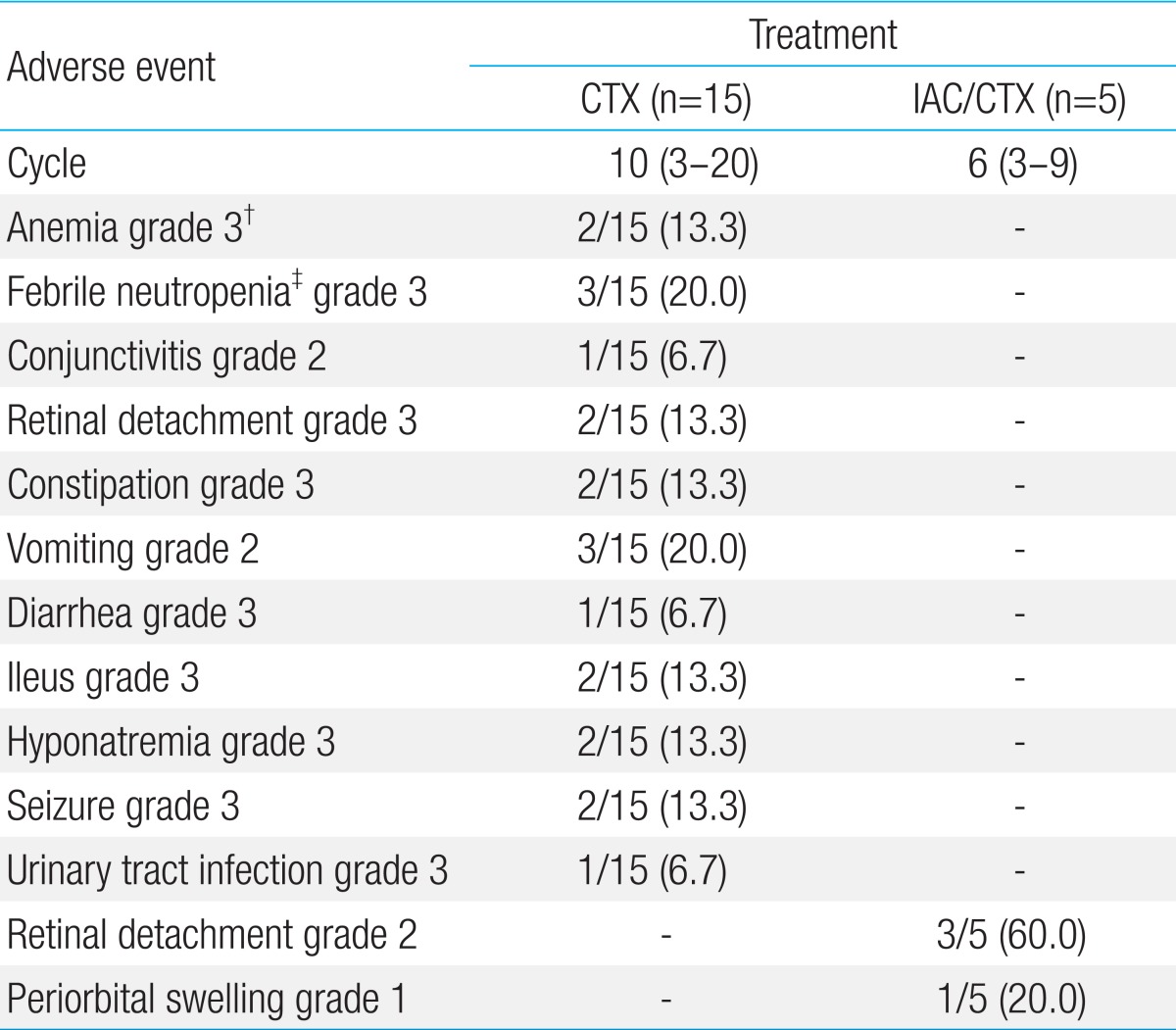
Grade 1 periorbital swelling was presented eye Aa in 2 weeks after IAC but it had resolved spontaneously. Grade 2 retinal detachment was shown in 60.0% (3/5). There was no grade 3 or higher neutropenia or other adverse events (Table 5).
4. EFS and PFS
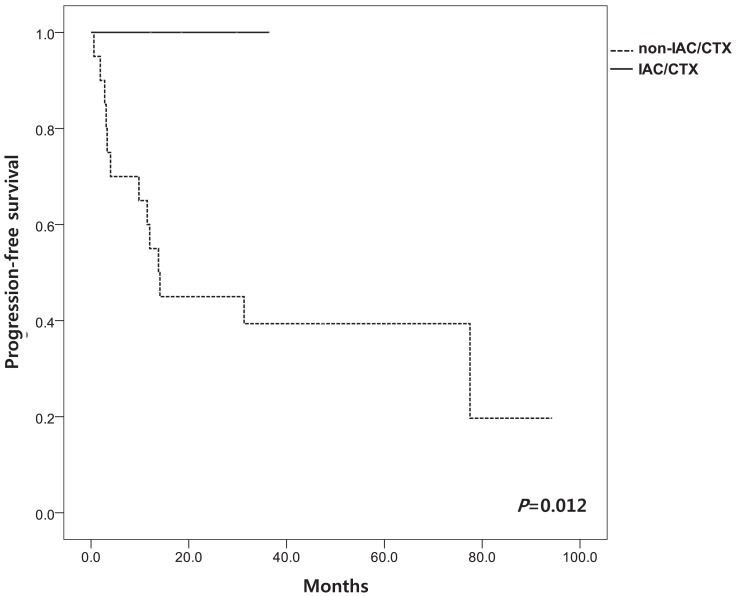
We evaluated EFS according to the stage and treatment modalities. Mean EFS according to RE stage at 60 months was 66±13.5% in early stage (I to III) and 33.0±12.1% in advanced stage (IV to V) (P=0.237) (Fig. 1). Among patients in non-IAC/CTX group, the mean EFS was 67.5±12.9% in early stage (I to III) and 33.1±9.2% in advanced stage (IV to V) (P=0.028). EFS at 36 months of IAC/CTX group was significantly higher than in non-IAC/CTX group (100.0% vs. 40.0±31.2%, P=0.016) (Fig. 2). PFS at 36 months was 100% in IAC/CTX and 35.0±9.8% in non-IAC/CTX (P=0.012) (Fig. 3).
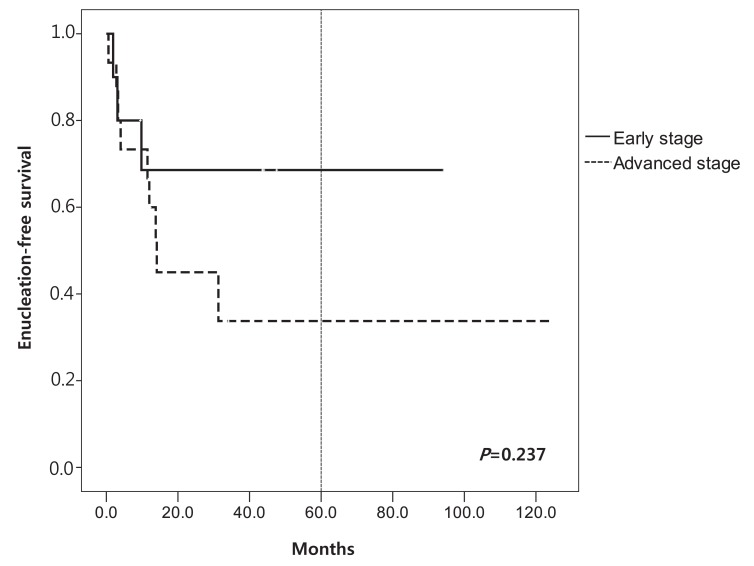
Enucleation-free survival according to Reese Ellsworth (RE) stage. RE stages I-III are described as "early stage" and stages IV-V are described as "advanced stage".
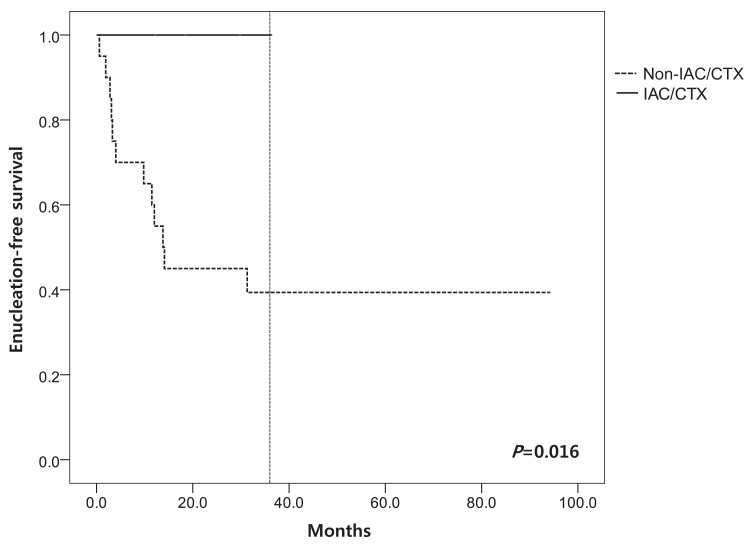
Enucleation-free survival according to type of treatment. IAC/CTX, alternative combined approach or intra-arterial chemotherapy.
Discussion
In order to preserve eyes and eye sight, treatment other than enucleation and EBRT have been tried. Systemic chemotherapy is preferred to the enucleation and EBRT, because those approaches cause eye globe loss and other significant ocular and systemic complications3). Unilateral RB is usually massive and has higher need for enucleation. It is possible to preserve eye sight when the size is small. In a study with 30 patients, 11% of RE stage II or III, 60% of stage IV, and 100% of stage V required enucleation or EBRT13). Several recent studies have shown that multi agent systemic chemotherapy with conjugation of focal treatment achieved 90% of radiation and EFS for early stage RB (RE stage I to III) and 30% for more advanced RB (RE stage IV to V)1). In this study, mean EFS according to RE stage at 60 months was 66.0% in early stage (I to III) and 33.0% in advanced stage (IV to V). In non-IAC/CTX group (traditional treatment group), the efficacy of the CTX in advanced RE stage was extremely unfavorable; 81.1% (9/11) was enucleated. EFS at 36 months in IAC/CTX group was 100% and all eyes were preserved even if 80% of eyes (4/5) were in advanced stage (RE stage V). It means that alternate IAC and chemotherapy approach improved EFS than other modalities.
In the past 5 years, IAC became promising approach for eye preservation and avoidance of chemotherapy complications. It has significantly reduced the enucleation even in the advanced intraocular RB (RE stage Va and Vb) and with minimized toxicity of systemic chemotherapy2). At first, IAC was tried as the second line treatment. A second line IAC study of 15 eyes of RB with retinal detachment showed, tumor regression in 27.0% (4/15). Eye globe was preserved in 71.0% (5/7) with total retinal detachment and 75% (6/8) with partial retinal detachment14). IAC has become the primary treatment of advanced stage in RB4,5). A large study of IAC for eyes with stage V showed EFS at 2 years was 80.0% as the primary treatment, and 51.5% as the second line treatment15).
IAC/CTX approach was recently adopted in this institution as the primary treatment for RB. Many studies showed efficacy of adjuvant CTX after enucleation for the patients with high risk factors such as involvement of anterior segment, surgical margin of optic nerve, postlaminar optic nerve, and choroid plexus6,13,16,17). Because there is a difficulty to determine exact metastatic risk before the enucleation and pathologic evaluation, we performed IAC/CTX for advanced RE stage patients. This combined approach is the unique; most of articles on the IAC approach gave IAC only as the first line or second line treatment for RB.
Generally, IAC do not have significant adverse events. Most of the studies report no systemic toxic effects of infused chemotherapeutic agents via arter. Local complication might occur in infused eyes14). Bianciotto et al.18) also reported vascular abnormalities observed through fluorescence angiography such as ophthalmic artery occlusion, spasm, and branch retinal artery stenosis. Despite of local complications, retinal functions are well preserved1,2,15,18-20).
Only few adverse events were observed in IAC/CTX group in our study. There were no grade 3 or 4 adverse events. In comparison to patients receiving only CTX, the number of cycles of chemotherapy in IAC/CTX group was less than in non-IAC/CTX group (median of 6 cycles [range, 3 to 9 cycles] vs.10 cycles [range, 3 to 20 cycles], P=0.083). The fewer cycles of CTX in IAC/CTX group may explain minimal adverse event in the group comparing with non-IAC/CTX group15,19).
This study but has some limitations. The number of IAC/CTX group was small and follow-up duration was relatively shorter than other groups. In order to confirm the long term efficacy of combined approach for metastasis prevention, we need at least 5 to 10 years of follow-up taking the median survival of RB patients into consideration. ER was lower and EFS was higher in IAC/CTX group for advanced stage but the interaction between stage and ER/EFS could not be assessed because the number of patients in IAC/CTX group was limited.
In summary, IAC/CTX approach was the effective treatment for preservation of eye globes. The IAC/CTX approach is another choice for the treatment of RB and the approach could be delivered safely without significant adverse events.
Notes
No potential conflict of interest relevant to this article was reported.