Introduction
In spite of extensive research and development in the understanding and treatment of neonatal sepsis, it continues to be the one of the most common causes of neonatal morbidity and mortality
1,
2). Early identification of neonatal sepsis is difficult because of the nonspecific or minimal clinical presentations. However, the clinical course of neonatal sepsis can be fulminant within hours of onset
3), and infection in the newborn period is associated with 10% of neonatal deaths
4). Thus, it is extremely important to make an early and accurate identification of neonatal sepsis for prompt antimicrobial therapy and better outcomes.
The current gold standard for confirming diagnosis of neonatal sepsis is blood culture. However, blood culture results are not available for 48 hours after starting the culture, and if blood cultures are drawn after administration of antibiotics, growth of microorganisms can be suppressed
5). Hence, a reliable inflammatory marker or set of markers is required for prompt and accurate identification of neonatal sepsis, so that delayed or unnecessary treatment can be avoided.
C-reactive protein (CRP) is a conventional inflammatory marker as a kind of acute phase protein. Concentrations of CRP increase at around 24 hours after onset of infection, peak between 36 and 50 hours and remain elevated throughout infection
6). CRP necessarily has a limitation for the early identification of infection within 24 hours of onset; therefore, serial measures are required 24 hours later for accurate detection of infection
3). Currently, in most neonatal intensive care units (NICUs) in Korea, CRP is practically used as an inflammatory marker, and a more accurate diagnostic maker is required on clinical base for better care of the newborns.
Recently, attention has been directed to leukocyte cell surface antigens as diagnostic markers of neonatal sepsis
7). Neutrophil surface marker, CD64 has high affinity to the Fc portion of immunoglobulin G (IgG) and is expressed and upregulated by bacterial or endotoxin interaction
8). Up until now, there have been several reports to show CD64's utility as an inflammatory marker for detection of neonatal infection including pneumonia, septicemia, necrotizing enterocolitis (NEC), meningitis and peritonitis in term or preterm newborns
9-
12). However, there have never been any reports to suggest that CD64 is a more superior diagnostic marker for neonatal sepsis compared to CRP as a single test regardless of the time of evaluation from onset of suspected sepsis in neonates and also there have never been any investigations to define CD64's utility for neonatal sepsis in Korea.
In this study, the authors investigated whether neutrophil CD64 is a more accurate diagnostic inflammatory marker compared to CRP as a single determination for the early detection of neonatal sepsis.
Discussion
An accurate inflammatory marker with high diagnostic sensitivity, specificity and NPV for neonatal sepsis would be a valuable tool for therapeutic decision-making and avoidance of unnecessary use of antibiotics
10,
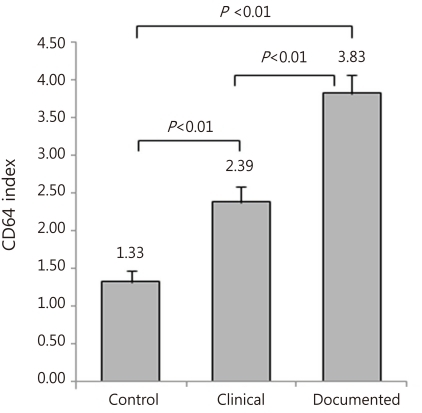
16). In the current study, CD64 as a leukocyte surface antigen was compared to the conventional and routine inflammatory marker, CRP. Our investigation showed that CD64 was a more superior diagnostic marker for the early detection of neonatal sepsis compared to CRP as a single determination.
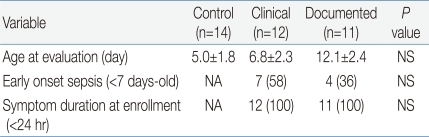
In the current study, we evaluated CD64 and CRP's diagnostic accuracy for only neonatal sepsis excluding local infections, unlikely to the previous studies
12). The reason for this is that local infections such as pneumonia, urinary tract infection, meningitis or NEC can be identified easily by other procedures including chest X-ray, urinalysis, spinal tap or other X-rays. However sepsis can be confirmed by only blood culture, that takes at least 48 hours for the result to be available. Hence, neonatal systemic infection is the most important cause of neonatal mortality and morbidity, and consequently, early identification of sepsis is still a critical issue in neonatal area.
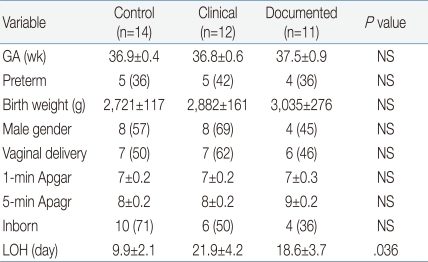
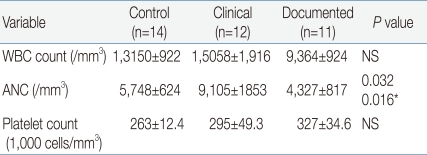
In terms of hematologic indices, our findings presented that only ANC significantly differed among the three groups. It has already been recognized that hematologic indices such as WBC and ratios alone cannot be confidently used as decision criteria for the identification of sepsis or for guiding antibiotic treatment
17). Our observations regarding WBC are congruent with a previous study showing variable sensitivity and specificity ranging from 17 to 90% for WBC and ratios
17). However, in general, an immature/total (I/T) ratio ≥0.2 is regarded as having high sensitivity, and leukopenia or neutropenia are considered to have high specificity
17-
19). Similarly, our observations showed low neutrophil counts (-4,500 cells/µL) in the documented group, which was a significantly lower value compared to the -9,000/µL observed in the clinical group. However, the sensitivity, specificity, PPV and NPV of ANC in our study ranged from 40 to 54%, which implicates that ANC alone cannot be a sensitive laboratory marker for neonatal sepsis. Our observation is partly supported by the previous study demonstrating WBC counts and ANCs can be affected by many factors besides infection, including age of the neonate, blood sampling method, mode of delivery, maternal hypertension and gender
20). In the present study, it was not available to assess the accuracy of I/T ratio as an inflammatory marker, because the immature neutrophils (band count) were not counted in the patients enrolled in the current study. Additionally, in the present study, it was not feasible to evaluate sensitivity and PPV of platelets, because there were no culture-confirmed cases with platelet counts less than 150,000/µL. However, similar to other hematologic indices, the NPV of platelets was only 50%.
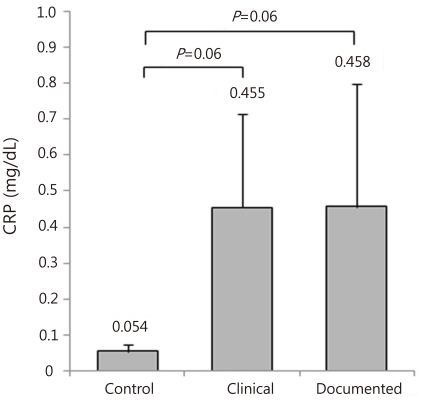
CRP goes up rapidly at around 24 hours after onset of infection. Consequently, CRP requires serial measures after 24 hours for accurate identification of sepsis, which is supported by a previous report showing that the sensitivity and NPV of CRP are enhanced higher when measured serially 24-hours later from admission, compared to a single measure taken at admission in premature infants
21).
CD64 is normally expressed in very low concentrations by unstimulated neutrophils, whereas it is considerably upregulated on the trigger of bacterial invasion
22). Namely, CD64 is the activation marker for neutrophils, and neutrophils react within an hour of acute inflammation
23). And CD64 has already been identified as a high-affinity Fc-gamma receptor of IgG antibody in the process of phagocytosis and intracellular killing of opsonized microbes
24,
25). The pathogens that require opsonization are encapsulated bacteria
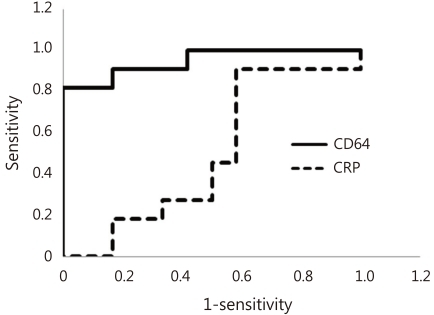
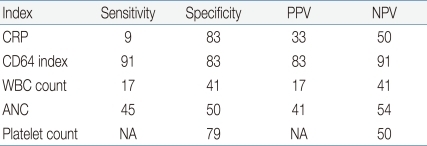
26), so that CD64 expression is not be related to viral infection. Based on these unique properties, CD64 is presumed to increase within an hour of bacterial infection, differently from CRP. In our study, symptom or sign duration of enrolled neonates were all within 24 hours, and the sensitivity, the specificity, the PPV and the NPV of CD64 were shown to be 91%, 83%, 83% and 91%, respectively, which were higher than those observed with CRP. Based on the fact that CRP usually goes up after 24 hours from onset of infection, these findings may be explained by the nature of CD64 to be capable of going up within one hour of infection. Although diagnostic utilities such as sensitivity, specificity, PPV and NPV, eventually determine the accuracy and usefulness of a clinical test, a high sensitivity and NPV would be the most important in neonatal infection because all genuinely infected newborns should be identified and treated
25). Additionally, acceptable specificity is considered to be >80% so that unnecessary use of antibiotics could be minimized
25). In the current study, we chose CD64's cutoff value of 3.0 to be the optimal point for a high sensitivity, specificity, PPV and NPV >80%. The cutoff value, 3.0, chosen in the present study, was close to 4.0 selected in the previous study
27), where CD64 index had a sensitivity of 80%, and a specificity of 79%, with a cutoff value of 4.02 for culture-positive sepsis episodes in neonates
27).
Additionally, the sensitivity and specificity of the CD64/CRP combination showed 100% and 66%, respectively. Hence the sensitivity showed similar to single CD64's (91%), whereas the specificity showed lower than 80% and rather lower than single CRP's. These results imply that the CD64/CRP combination could be efficacious in guiding decisions to start empirical antibiotic therapy similarly to CD64, while not be efficacious in guide decisions to withhold antibiotic therapy.
According to the previous evidence, the diagnostic accuracy of CD64 for sepsis was achieved 24 hours later in term newborns
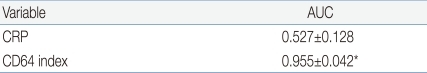
10). However the recent study showed that CD64 index measured within 24 hours from onset of suspected sepsis was reliable with high sensitivity, specificity, PPV and NPV for the early determination of sepsis, which indicates that CD64 is a useful laboratory marker as a single value for neonatal sepsis, regardless of the time from onset of suspected sepsis. Additionally, our present data showed that CD64 index has a significantly higher AUC (0.955) compared to CRP (0.527), with a cutoff value of 3.0 of CD64 and 1.0 mg/dL of CRP.
In the current study, coagulase-negative staphylococci have grown in six patients, and all these pathogens were identified within 24 hours from starting of the blood cultures. Based on the previous evidences showing that pediatric BAC-TEC system found 97% positive within 48 hours from starting of the blood culture, and microorganisms grown beyond 48 hours from starting of the blood cultures should mostly be caused by contamination
28), and in most circumstances, if blood culture results are not reported as positive by 48 hours, then empiric administration of antibiotics may be discontinued
14,
29,
30), coagulase-negative staphylococci grown in six patients enrolled in the study should be considered to be pathogens. Additionally, their clinical backgrounds such as prolonged total parenteral nutrition, suspicious NEC and infected epidermal cyst so on forth mentioned in the results should support that they are pathogens, not contaminants.
CD64 index has some general favorable characteristics; The expression of CD64 does not differ with age, as its expression only occurs upon cell activation
21) and is stable for more than 30 hours at room temperature
27). In addition to accuracy, the laboratory test for CD64 is rapid (<60 minutes) with the use of flow cytometry and requires minimal blood volume (<100 µL). In fact, in our study, no extra blood was obtained to calculate the CD64 index, as CBC samples proved sufficient. Hence, it is practical to obtain the CD64 index in a clinical setting. However, the critical issues such as cost and clinical settings should be considered carefully. For the current study, it cost approximately $16 to obtain one CD64 index, which was more expensive than CRP's. For the recent study, CD64 index were measured using a FACscan system established at the department of clinical diagnosis.
In conclusion, our preliminary study suggests that the CD64 index is a more reliable diagnostic measure for confirmed neonatal sepsis as a single value compared to CRP, and the CD64 index may be promise for replacing CRP. However, the important issues of cost and availability are required to be evaluated in routine clinical setting.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


