Introduction
Kawasaki disease (KD) is a systemic vasculitis syndrome that features a high fever in infants and children. In Korea, 105 out of 100,000 people contract it, and the incidence rate ranks second in the world after Japan1). Despite numerous studies that have been carried out since it was reported first in 1967, the cause has not yet been established. As the causative pathogen has not been identified and excessive immune activation is observed in almost all patients during the acute phase of KD, we believe that abnormal immune activation from a nonspecific infection might be a possible cause.
The immune mechanism must maintain a balance between activation and suppression; specifically, when an infection occurs, the immune system is activated to remove the pathogen, but also regulated to preclude damage to autologous tissue. In the human body, various cells participate in the regulation of the immune response. Although CD25 and the forkhead family transcription factor (Foxp3) are the most representative markers for the T regulatory cells (Tregs), they are also expressed in activated T cells. Foxp3+ T cells that do not express CD25 are unable to function properly as Tregs2). Approximately 30% of human CD4+ T cells express CD25 but only 1-3% express it at a high level, functioning as Tregs that suppress conventional T cell activation in a contact-dependent, dose-dependent, and antigen-nonspecific manner3, 4). In recent studies, dysregulation in CD4+ Tregs with relatively high levels of CD25 (CD4+CD25highFoxp3+ cells) has been demonstrated in various autoimmune diseases and cancer3, 5-7). As immunoregulatory abnormality may be related to the cause of KD, we investigated the profiles of Tregs in acute KD as well as the changes of relative ratio correlated to the clinical course of KD.
The purpose of this study was to investigate the roles of cells that express CD4, CD25, and Foxp3 markers in KD. Accordingly, the expression pattern was analyzed in patients with KD that showed excessive immune activation during the acute phase, and the changes were related to the progress of the disease.
Materials and methods
1. Materials
This study was performed on 17 patients diagnosed with KD between March and November, 2008 at Korea University Medical Center Ansan Hospital. The cases were characterized by a fever of more than 38.5Ōäā that continued for at least 5 days and was accompanied by at least four out of the following five symptoms: polymorphous rash, extremity changes, cervical lymphadenopathy, conjunctival injection, and distinctive oral manifestations. Eight patients satisfied all the diagnostic criteria. In the other nine patients where the clinical manifestations did not fulfill the diagnostic criteria and other diagnoses were excluded, symptoms that are frequently associated with KD and elevated inflammatory indices during the acute phase were considered to support the diagnosis of incomplete KD8, 9). These cases of incomplete KD were only included in this analysis if they showed a typical convalescent condition such as skin peeling. All 17 subjects were treated with intravenous immunoglobulin (IVIG, 2 g/kg) in addition to aspirin (50-80 mg/kg/day). Echocardiography was performed usually at 7-10 days after onset of fever and thereafter at monthly intervals for at least 2 months. During this period, patients exhibiting any coronary abnormality, regardless of later resolution, were counted as having a coronary abnormality. In accordance with recommendations in a report from a subcommittee of the Japanese Research Committee on KD in 1984, a coronary artery abnormality is defined as a coronary artery diameter of at least 3 mm (those aged less than 5-years-old) or 4 mm (those aged 5 and older), and 1.5 times larger than the diameter of the closest blood vessel and an inner surface irregularity of the blood vessel10).
2. Methods
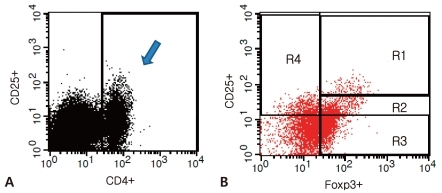
The peripheral blood of subjects was collected before the use of IVIG to bring down the fever and 2 days after defervescence, with the consent of their parents. After density-gradient centrifugation using a Ficoll centrifuges (Amersham Pharmacia, Biotech, Piscataway, NJ, USA), mononuclear cells were separated from peripheral blood. Mononuclear cells at a concentration of 1├Ś106/100 ┬ĄL phosphate-buffered saline were mixed with a cocktail of allophycocyanin (APC)-antihuman CD4 antibody and fluorescein isothiocyanate (FITC)-antihuman CD25 antibody (eBioscience, San Diego, CA, USA). The mixture was allowed to react at 4Ōäā for 30 min and was then rinsed with the phosphate-buffered saline. After 2 ┬ĄL of fixation and permeabilization solution was added, the mixture was allowed to further react at 4Ōäā for 30 min. Again, it was rinsed by the phosphate-buffered saline. In the same way, phycoerythin (PE)-antihuman Foxp3 antibody (eBioscience, San Diego, CA, USA) was administered, marked, and rinsed. The subjects were classified into four groups, i.e., a control group, a group of mononuclear cells marked by antihuman CD4 and CD25 antibodies, a group marked by the antihuman Foxp3 antibody, and a group marked by both antihuman CD4 and CD25 antibodies and antihuman Foxp3 antibodies. The subjects were then analyzed by the use of flow cytometry (FACSCalibur, CellQuest software, Becton Dickinson Immunocytometry System, San Jose, CA, USA)11). Expression of CD25 and Foxp3 were determined in gated CD4+ T cells, and consequently each cell population was expressed as the percent of CD4+ T cells. Foxp3+ T cells, based on their CD25 expression, were divided into three populations: CD4+CD25-Foxp3+ T cells, CD4+CD25lowFoxp3+ T cells, and CD4+CD25highFoxp3+ T cells (Fig. 1).
For statistical analysis, SPSS (version 12.0, SPSS Inc., Chicago, IL, USA) was used with the paired t-test. Only cases in which the P value was less than 0.05 were considered to be significant, and the data were expressed in 'percentage (%)' or 'the mean┬▒the standard deviation (SD)'.
Results
1. Characteristics of subjects
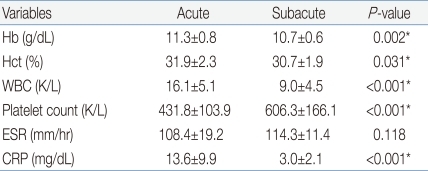
The average age of the 17 subjects was 30.8┬▒15.8 months, and the ratio of males to females was 10:7. Their fevers were lowered after a single dose of IVIG (2 g/kg). After the administration of IVIG, the febrile phase lasted 1.4┬▒1.1 days on the average, and the whole period of fever was 6.9┬▒1.4 days. Two out of 17 subjects were diagnosed with coronary artery abnormalities, and on the echocardiographic follow-up performed 1 month later, both of them showed regression. The blood test showed that the hemoglobin and leukocyte levels decreased after defervescence but the platelet level increased. The erythrocyte sedimentation rate (ESR) increased but was not significant. C-reactive protein decreased remarkably after defervescence (Table 1).
2. The altered percentages of CD4+Foxp3+ and CD4+Foxp3- T cells in KD
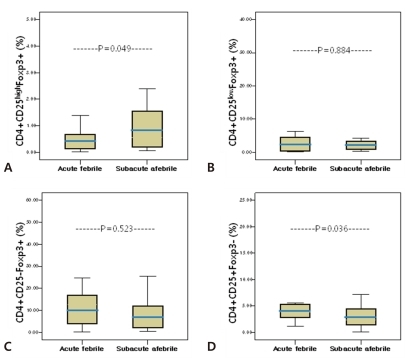
The percentage of circulating CD4+CD25highFoxp3+ T cells among the total CD4+T cells was significantly higher during the subacute afebrile phase than during the acute febrile phase (1.10┬▒1.22% vs 0.55┬▒0.53%, P=0.049) (Fig. 2A). In contrast, the percentages of CD4+CD25lowFoxp3+ T cell and CD4+CD25-Foxp3+ T cell populations did not show significant change (Fig. 2B, C). The proportion of CD4+CD25lowFoxp3+ T cells in both phases was similar (acute febrile phase, 4.52┬▒8.83%, subacute afebrile phase, 4.11┬▒5.69%, P=0.884). Although the percentage of CD4+CD25-Foxp3+ T cells was decreased during the subacute afebrile phase, it was not significant (10.86┬▒14.32% vs 12.69┬▒11.12%, P=0.523). Furthermore, the percentage of CD25+Foxp3- T cells was decreased in the subacute afebrile phase compared to the acute febrile phase (2.96┬▒1.95% vs 5.64┬▒5.69%, P=0.036) (Fig. 2D).
3. The altered ratio of CD4+Foxp3+ T cell subsets to CD4+CD25+Foxp3- T cells
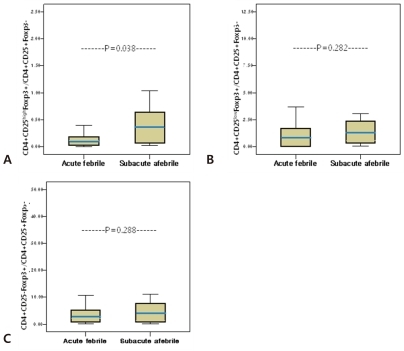
The relative ratio of each subset of CD4+Foxp3+ T cells to CD4+CD25+Foxp3- T cells, which shows the balance between two groups, was determined (Fig. 3). The ratio of CD4+CD25highFoxp3+ T cells to CD4+CD25+Foxp3- T cells showed a significant low point during the acute febrile phase (0.13┬▒0.13% vs 0.45┬▒0.57%, P=0.038) (Fig. 3A) However, there were no significant differences in the ratios of CD4+CD25lowFoxp3+ T cells and CD4+CD25-Foxp3+ T cells to CD4+CD25+Foxp3- T cells between phases (acute febrile phase, 1.58┬▒2.84%, subacute afebrile phase, 6.11┬▒16.21%, P=0.282; acute febrile phase, 4.82┬▒4.81%, subacute afebrile phase, 32.18┬▒102.81%, P=0.288, Fig. 3B and 3C, respectively).
Discussion
CD25 and Foxp3 are the principal markers of Tregs but are simultaneously expressed in activated T cells; for that reason it is not easy to distinguish Tregs from activated T cells. It is not yet clear whether all Tregs express Foxp3 and whether all Foxp3-expressing cells are Tregs. It is impossible to analyze the function of Foxp3+ cells, because it is expressed intracellularly and its identification requires the fixation and permeabilization of cells. In this study, CD4+Foxp3+ Tregs were divided into CD25high T cells, CD25low T cells, and CD25- T cells according to the degree of expression of CD25, and the numerical pattern of expression in these cells was correlated with the clinical courses of KD. During the acute phase, the proportion of CD4+CD25highFoxp3+ T cells was lower than that during the subacute phase, which clinically corresponded to their suppressive regulatory function. However, in the case of CD25low or CD25- expression, the proportion of Foxp3+ cells decreased, instead, during the subacute phase compared to the acute phase, although it was not significant. These results imply that the function of Foxp3- expressing cells as Tregs needs to be further examined. There has been considerable controversy over the role of CD25low T cells and CD4+CD25-foxp3+ T cells. Recently, it was suggested that these cells reflect the self-limiting nature of KD, acting as induced Tregs that have immunoregulatory function12), but on the other hand it has been reported that these cells are like Tregs from the viewpoint of phenotype, but are more like activated T cells on the basis of function13, 14). According to studies on systemic lupus erythematosus (SLE) patients, CD4+CD25highFoxp3+ T cells decreased during the acute period, but CD4+CD25lowFoxp3+T cells and CD4+CD25-Foxp3+ T cells increased with the severity of the disease7). In the process of reactivation, CD25-Foxp3+ T cells re-express CD25 and thus function as a peripheral reservoir of CD25+Foxp3+ Tregs and restricted regulatory cells2, 15). Consequently, it is supposed that at the very least, CD25-Foxp3+ T cells are not fully functional Tregs. Furthermore, the relative increase of CD25+Foxp3- T cells during the acute phase might reflect immunoactivation in KD through their action as T effector cells. Considering the importance of homeostatic balance in immunoregulation, it is meaningful to measure not only the change in level of each Tregs, but also the ratio of CD4+CD25+Foxp3- T cells to each type of Foxp3+ T cells. In patients with SLE, the percentages of CD4+CD25highFoxp3+ T cells, CD4+CD25lowFoxp3+ T cells, and CD4+CD25+Foxp3- T cells increase in inverse proportion to the severity of the disease as compared to healthy controls7, 16). In our study, CD4+CD25+Foxp3- T cells decreased but CD4+CD25highFoxp3+ T cells increased during the subacute afebrile phase of KD, resulting in a significantly heightened ratio that correlated with a decrease in the severity of the disease. The ratio of CD4+CD25lowFoxp3+ T cells to CD4+CD25+Foxp3- T cells was also heightened but was not significant.
All of the subjects were treated with IVIG, and a series of changes were expected after defervescence. One working mechanism of IVIG is to bind to cells that secrete inflammatory cytokines and chemokines and suppress the secretion17). In addition, IVIG directly recovers the suppressive function of Tregs. IVIG binds more strongly to Tregs than to conventional T cells and induces an increase in Tregs both functionally and numerically17, 18). Treg levels increased in the spleen after IVIG, caused by the expansion of preexisting Tregs rather than de novo generation18). In addition to the induction of expression of Foxp3, as well as to the secretion of immunosuppressive TGF-╬▓ and IL-10, IVIG increases the proportion of CD4+CD25highFoxp3+ T cells19). Corticosteroid and infliximab, which are cited as alternative treatments for intractable KD, increase the level of Tregs in some cases20, 21). In the mean time, a therapy to directly induce Tregs in human patients suffering from allergies, by the use of specific 'probiotic' bacteria to build autoimmunity22, 23), could serve as a model for a possible alternative treatment for KD.
In many cases, serious coronary artery complications are related to a lengthy inflammatory condition. Brown et al.4) who performed autopsies on eight children that died during the acute phase of KD reported an increase in the transmural infiltration of proinflammatory T cells such as CD8+cytotoxic T cells and CD4+ T helper 1 cells. In other studies, coronary artery complications readily occurred upon increased cytokine secretion from T cell activation24-26). In our study, two subjects had coronary artery complications. Although these patients did not show a remarkable decrease of CD4+CD25highFoxp3+ T cells or a remarkable increase of CD4+CD25+Foxp3- T cells during the acute febrile phase, the ratio of CD4+CD25highFoxp3+ T cells to CD25+Foxp3- T cells was relatively low and CD4+CD25highFoxp3+ T cells were not conspicuously recovered during the subacute afebrile phase (data not shown). More human studies are needed to more fully elucidate the role of CD4+CD25highFoxp3+T cells in the development of coronary abnormalities.
In conclusion, decreased CD4+CD25highFoxp3+ T cells, increased CD4+CD25+Foxp3- T cells, and/or an imbalanced ratio of CD25highFoxp3+ T cells to CD4+CD25+Foxp3- T cells might be related to the immune activation that occurs during the acute phase of KD. Reversal of these parameters which was observed during the subacute afebrile phase, is likely a result of the treatment with IVIG. To define the roles of Tregs in the pathogenesis of KD, further studies that include normal controls as well as other febrile disease controls are needed.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


