Introduction
Bronchopulmonary dysplasia (BPD) that develops after surfactant and non-invasive ventilation therapy is characterized by alveolar developmental arrest. In recent times, most preterm infants with BPD have been born between the late canalicular and early saccular stages, during which active acini and alveolar sac formation occurs. Preterm infants born in this period have to breathe with structurally and functionally immature lungs. In a majority of such cases, ventilation assistance and supplemental oxygen are required for survival. Lung development involves the temporal and spatial coordination of a number of transcription and growth factors and cell-cell interactions. This precise developmental process is disrupted in prematurely born infants by various means, including hyperoxia, mechanical stretch, and inflammation1-3).
Until the saccular stage, alveolar sacs are made by dichotomous branching tubules4, 5). Further extension of the gas exchange surface from the alveolar sacs occurs through a different mechanism termed alveolar septation. Saccular walls are subdivided by the protrusion of secondary septa that grow perpendicularly into the air space6). Lung interstitial cells play crucial roles in secondary septa formation, and their number markedly increases during alveolarization and decreases thereafter7).
Elastic fibers
The lung consists of 3 interconnected elastic fiber systems8). Axial elastic fibers originate from the bronchiolar wall and form an outline of alveolar ducts. Peripheral elastic fibers are connected to the pleura and penetrate the area beneath alveolar acini. Septal elastic fibers appear as saccules and alveoli forms and are anchored to both axial and peripheral elastic fiber systems9, 10). Elastin is a very stable molecule and once interconnection of these elastic fiber systems is completed, the components retain their configuration11).
Lung fibroblasts
Two subsets of interstitial cells exist during lung development, including myofibroblasts or nonlipid lung interstitial cells and lipofibroblasts or lipid interstitial cells12), and appear to be derived from the same mesenchymal cells. However, the cells show varying growth rates at any age and are hypothesized to be separate populations13, 14). Lipofibroblasts contain lipid droplets containing triglycerides, cholesterol esters, and retinyl esters, and are present at the base of elongating septa during alveolar septation15, 16). Lipofibroblasts are thought to supply triglycerides for surfactant phospholipid synthesis15). More importantly, these cells are a source of retinoic acid, which has a major role in alveolarization.
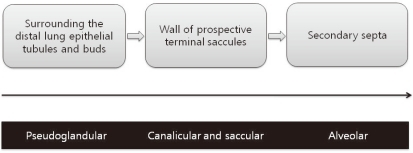
Myofibroblasts lack lipid droplets and express smooth muscle actin. They are contractile cells and the source of septal elastin17). Myofibroblasts are located in developing secondary crests and at septal tips during the alveolar stage of lung development and are thus called alveolar myofibroblasts18). They are dual positive for elastin and smooth muscle actin in immunostaining assays, suggesting a role in elastin formation19). Alveolar myofibroblasts have morphologic and biochemical characteristics intermediate to fibroblasts and smooth muscle cells. During the pseudogladular stage of lung development, progenitors of alveolar myofibroblasts exist as a population of lung mesenchymal cells expressing platelet derived growth factor receptor (PDGFR)-α around the distal lung epithelial tubules and buds20). During the canalicular and saccular stages, these PDGFR-α-positive cells spread from their location around the distal epithelial buds to the walls of prospective terminal saccules to eventually become alveolar myofibroblasts (Fig. 1). Alveolar myofibroblasts are critical for secondary septa formation. Their absence is associated with a lack of secondary septation and the alveolarization failure21). Dysregulated myofibroblast development has been implicated in BPD22). Alveolar myofibroblasts are abundant during alveolarization, but are absent in adult lungs23). Because an excess of myofibroblasts is implicated in fibrotic diseases, alveolar myofibroblast disappearance following alveolarization completion may be critical. However, the regulatory mechanism of survival during alveolarization and subsequent disappearance of alveolar myofibroblasts is poorly understood.
Elastic fiber formation and alveolar septation
The force necessary for lifting the alveolar crest from the primary septa wall is thought to be produced by septal elastic fibers. First, elastic fibers accumulate in the thickened area of primary septa (septal crest). Secondary, this thickened area grows into secondary crest that protrudes perpendicularly from the saccular wall into air space. Finally, secondary crest further extends into secondary septa. During this process, elastic fiber deposits are always located at the fore-end of developing secondary septa (Fig. 2). This feature is universally observed in all species, including humans26). Mechanical stress to elastic fibers located along saccular wall bends is believed to cause the fibers to protrude from the saccular walls, leading to new septum formation6). However, another theory involves a repulsive signal to epithelial cells in the primary septum may push the secondary septum into air space. In this theory, myofibroblasts simply follow epithelial cells27).
The essential role of elastic fibers in septation is supported by various studies. Elastin expression in cultured fibroblasts isolated from rat lung at various postnatal ages is consistent with the degree of septation28).
Hyperoxia that inhibits alveolarization also significantly decreases elastin expression29). In vivo studies demonstrate that postnatal lysyl oxidase (LOX) inhibition or inactivation of the LOX gene, which interferes with elastin and collagen synthesis, leads to alveolar septation impairment30, 31). In various pathological conditions leading to impaired alveolarization, such as in a premature lamb BPD model32), a transgenic arrested alveolarization model in mice33), mechanically ventilated newborn mice34), and congenital diaphragmatic hernia35), elastin synthesis was consistently disrupted. Thick and tortuous elastic fibers formed disorganized meshwork along alveolar walls instead of their normal location at septal tips.
Control of myofibroblast differentiation and migration
Transforming growth factor β1 (TGF-β1) plays a crucial role in lung myofibroblast differentiation36). In vitro TGF-β1 stimulates smooth muscle actin and tropoelastin expression in lung fibroblasts37). In rats, active TGF-β1 and TGF-β receptors increase before alveolar septation. Mice devoid of smad3, a major intracellular downstream signal transducer in the TGF-β1 pathway, show inhibited alveolarization with concomitant decreased expression of tropoelastin38, 39). As alveolar septation begins, expression and localization of TGF-β family members and bone morphogenetic proteins (BMPs) concurrently are changed40). This indicates that these proteins are involved in alveolar septation. TGF-β also plays a role in lung fibrosis development41). TGF-β is also increased in preterm infants with BPD and excessive TGF-β expression is associated with alveolarization inhibition in neonatal animals42, 43). Hyperoxia increases TGF-β expression and induces excessive myofibroblast differentiation, but impairs BMP signaling44, 45). These findings suggest that precise balance of control between TGF-β and BMP signaling is essential to alveolar septation.
Platelet-derived growth factor A (PDGF-A) also plays a crucial role in alveolar septation. Disruption of the PDGF-A gene results in failure of elastic fiber deposition in saccular walls and secondary septation. PDGF-A is produced by epithelial cells and acts as a chemoattractant, allowing myofibroblast precursors expressing PDGFRα to migrate to peripheral locations46). Thus, PDGFRα expression by alveolar myofibroblasts is not only a marker, but also has functional consequences.
Insulin-like growth factor I (IGF-I) released by epithelial cells is involved in myofibroblast proliferation, differentiation, and migration. Exogenous IGF-I enhanced migration and proliferation of fibroblasts in vitro and the extent of alveolar development was well correlated with IGF-I levels in animal models47, 48). IGF-I increases α-smooth muscle actin expression and collagen synthesis in developing lung fibroblasts49).
Fibroblast growth factor (FGF) signaling in alveolarization
Fibroblast growth factors (FGFs) play crucial roles in various steps of lung development50) and are mediated by tyrosine kinase receptors (FGFR1-4)51). Alveolar septation coincides with increased FGFR3 and FGFR4 expression52). In mice devoid of both receptors, alveolar septation did not occur, but myofibroblasts were present and elastin formation occurred. However, elastin deposition occurred at atypical locations other than tip of growing septa and elastin formation failed to cease and contined to accumulate into adulthood53). FGF2 down-regulates elastin synthesis and LOX activity and is thus considered involved in septal elastogenesis arrest54, 55). Unlike FGF2, FGF18 appears to be involved in elastogenesis. An increased FGF18 level coincident with the beginning of alveolar septation has been observed in human fetal lung and postnatal rat lung56). FGF18 coordinately up-regulates tropoelastin and LOX expression in isolated rat lung fibroblasts56). FGF18 expression markedly decreases in alveolarization arrest animal models induced by hyperoxia57). FGF18 expression is also reduced in the hypoplastic lung of human fetuses with congenital diaphragmatic hernia58). Enhancement of lung growth coincides with the restoration of FGF18 expression, elastic fiber density and location, and alveolar septation58). FGF7 (keratinocyte growth factor) is expressed exclusively in interstitial cells, but its specific receptor, FGFR2IIIb, is found only in epithelial cells59, 60). This implies that FGF7 may be involved in pulmonary mesenchymal-epithelial interactions. Recently, FGF7 was reported to affect alveolar septation by enhancing vascular bed growth61). FGF9 controls mesenchymal cell proliferation in the prenatal lung and FGF9 signaling is necessary for distal lung capillary development62, 63).
Alveolarization-related gene expression in myofibroblasts
It can be assumed that genes predominantly expressed in newly forming septa are involved in alveolarization. Galectin 1, a β-galactosidase-binding protein involved in regulation of cell proliferation, differentiation, and apoptosis, was concentrated in myofibroblasts located at the septal tip with peak level at the time of active alveolar septation64). This suggests an important role of galectin-1 in alveolarization. In fibroblasts isolated from developing rat lungs, 2 groups of genes showed markedly opposite expression patterns before, during, and after alveolar septation65). Genes up-regulated during septation and down-regulated afterward are the transcription factors Hoxa2, 4, 5, and retinoid X receptor γ (RXR γ), and 3 genes involved in Wnt signaling, Wnt5a, Norrie disease protein (Ndp), and the receptor frizzled 1 (Fzd1). Their protein products were detected in the septal crest and tips of growing septa. Fzd1 is reported to be involved in myofibroblast proliferation and differentiation66). Genes down-regulated during septation and up-regulated thereafter include cartilage oligomeric protein, osteopontin, osteoactivin, TnX, and schlafen 4. Their expression profiles suggest involvement in the alveolar wall thinning and microvascular maturation phases. Notably, expression of genes up- and down-regulated during septation decreased or was enhanced, respectively, in arrested alveolarization models65). This observation suggests that alveolarization not only involves up-regulation of specific genes but also requires down-regulation of other sets of genes.
Conclusion
Lung interstitial cells, especially alveolar myofibroblasts, are essential for secondary septation, a critical process in alveolarization. The locations of myofibroblasts during lung development and their disappearance after the completion of alveolarization imply their crucial role in the formation of new gas exchange units. The differentiation and migration of myofibroblasts is strictly controlled by various mediators and genes. Thus, any disruption in control pathways may lead to abnormal alveolarization. To increase the understanding of BPD, a representative disorder of disrupted alveolarization, fine mechanisms involved in survival, cell-cell interactions, and disappearance of lung myofibroblasts should be searched.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


