Introduction
Tobacco smoke contains approximately 4,000 toxic chemicals, including oxidative gases, heavy metals, cyanide, and at least 50 carcinogens. Tobacco use is the most preventable cause of death and the most serious risk factor for cancer. Currently, 1.3 billion people smoke or use tobacco, and nearly 5 million worldwide die of diseases associated with tobacco smoke each year1). Environmental tobacco smoke (ETS) consists of particles much smaller than those in mainstream smoke, and therefore has greater penetrability to the airways of children.
Passive exposure to tobacco smoke significantly contributes to morbidity and mortality in children. Children, in particular, seem to be the most susceptible population to the harmful effects of ETS2). Children are exposed to tobacco smoke not only in their homes but also in schools, restaurants, child-care settings, cars, buses, and other public places. The home is the greatest single source of ETS for children. Paternal smoking inside the home leads to significant maternal and fetal exposure to ETS and may subsequently affect fetal health3).
ETS is defined as tobacco smoke produced by an active smoker both from the exhalation of smoked tobacco and by the burning end of the cigarette, which is inhaled by nonsmokers4). Exposure to ETS among children in their homes have been reported to vary from 27.6% in Africa, 34.3% in Southeast Asia, 50.6% in Western Pacific, and up to 77.8% in Europe5). ETS have been associated with adverse effects on pediatric health, including preterm birth, intrauterine growth retardation, perinatal mortality, respiratory illness, neurobehavioral problems, and decreased performance in school.
In this review, we discuss the effects of ETS on pediatric health and the technical issues concerning methods used for estimating ETS.
Pathophysiology of ETS
Fetuses and children are more vulnerable to the harmful effects of ETS because of their unique manner of exposure and their dynamic developmental physiology. Exposure to ETS during pregnancy can be fatal to growing embryos. Smoke condensates may induce the remodeling of embryonic vasculature, leading to various pathologies6). Exposure of the fetus to toxicants, which enter through the umbilical cord of the mother exposed to tobacco smoke, is associated with altered alveolar and respiratory bronchiole growth and development7).
Children are far more sensitive than adults to toxic chemicals in the environment. Proportional tobody weight, children drink more water, eat more food, and breathe more air than adults8). The physical attributes of children also cause them to live closer to the ground than adults do, which increases their exposure to toxins in dust, soil, and carpets. Children's ability to detoxify differ from that of adults owing to their physiological status and the immaturity of their enzyme systems and clearance mechanisms9). Children not only have higher metabolic rates but also inhale much greater volumes of air per kilogram body weight than adults (inhalation of 0.53 m3·kg-1·day-1 of air vs. inhalation of 0.2 m3·kg-1·day-1, respectively)2). The additional factor that increases children's exposure to ETS is their tendency to often sit closer to their parents, family members, or caregivers, which brings them closer to the source of pollutants than other passive smokers. Thus, the harmful effects of ETS on health are more severe in children than in adults.
ETS and respiratory health in infants and children
Prenatal maternal smoking and postnatal ETS lead to a dose-dependent decrease in lung function and respiratory morbidity in infants and children. Exposure of children to ETS in the home increases the incidence of middle ear disease10), asthma, wheeze, cough, bronchitis, bronchiolitis, pneumonia, and impaired pulmonary function.
A recent review by Stocks and Dezateux11) reveals that many studies have demonstrated a reduction in forced expiratory flows in infants exposed to parental smoking. Although several studies have shown reduced lung function in the early months of life in infants exposed passively to tobacco smoke during pregnancy12-15), it is difficult to separate the effects of prenatal and postnatal exposure on lung function during infancy. Current passive smoking is associated with effects ranging from -0.5% forced expiratory volume in 1 second to -2% maximal expiratory flow (MEF50)16). The effect of prenatal exposure to tobacco smoke can last at least up to adolescence17). Mannino et al.18), in the United States (US), also found a strong association between higher serum cotinine levels and worse lung function among children aged 8 to 16 years. A population-based cohort study (n=1,781) from 6 US sites in 2000 to 2006 found that childhood ETS exposure from 2 or more smokers compared with none is associated with early emphysema in adulthood after adjustment for demographic, anthropometric, parental, and participant characteristics19).
Many studies consistently demonstrated that parental smoking has an important impact on asthma and wheezing illnesses in infants and children. Exposure to ETS is associated with increased wheezing illnesses and increased symptoms in asthmatics20,21). A study conducted in 3-year-old children who were exposed both prenatally and postnatally to ETS reported increased prevalence of wheeze (odds ratio [OR], 1.14) when compared with children born to nonsmoking parents22). Maternal smoking increased the risk of asthma (adjusted OR, 1.35 for high exposure) during the first 7 years of life, in a dose-dependent manner according to the mothers' smoking rates23). Studies on the association of parental smoking with respiratory symptoms in school-aged children report an OR for asthma of 1.21, wheeze 1.40, and cough 1.35 for either parent smoking24,25).
In a review by Strachan and Cook26), increases in lower respiratory illness (LRI) were found to be associated with maternal smoking. Hospital admission with bronchiolitis was up to 3 times more likely with exposure to ETS. An OR of 1.69 for LRI was found for both parents smoking versus neither smoking.
Recently, gender-specific differences in the effects of ETS were noticed. Among children with allergic predisposition, more associations between ETS exposure and respiratory symptoms and diseases were detected in girls27).
ETS and infection in children
Exposure to ETS has been shown to be associated with increased prevalence of upper respiratory tract infections2). Several studies have also shown that parental smoking is associated with an increased incidence of both upper and lower respiratory tract infections (LRTI)28,29).
Gürkan et al.30) reported that children with respiratory syncytial virus bronchiolitis were found to have higher levels of cotinine when either the mother or both of the parents smoked, than do children with nonsmoking parents. This finding implies that the risk of acute respiratory infection can be elevated by heavy exposure to cigarette smoke. Passive smoking may play an important role in the development of respiratory infections and can cause airway inflammation in children with existing LRTI28).
A meta-analysis confirmed that ETS exposure at home has a major influence on the risk of LRI in infants, especially bronchiolitis31). Smoking by either parent or other household members significantly increased the risk of LRI (OR, 1.54 for any household member smoking).
A US National Cancer Institute report concluded that ETS exposure is strongly associated with otitis media, especially among children younger than 2 years32).
The mechanism by which ETS may be related to these infections is not entirely clear, but may be through suppression or modulation of the immune system, enhancement of bacterial adherence factors, or impairment of the mucociliary apparatus of the respiratory tract, or possibly through enhancement of toxicity of low levels of certain toxins that are not easily detected by conventional means29).
ETS and cardiovascular health in children
Environmental and genetic factors are the main determinants of cardiovascular disease risk factor clustering in families. ETS exposure may be associated with the progression of an index of atherosclerosis. Long-term exposure to ETS creates a state of permanent inflammation and an imbalance in the lipid profile that leads to lipid accumulation in the blood vessels of the heart and aorta33).
Children with long-term exposure to ETS may have an elevated risk for the development of premature coronary artery disease34). Permanent vascular damage is partly attributable to familial tobacco smoke exposure, an association that might be initiated in gestation. From a cohort of 732 young adults, birth data were collected and common carotid artery intima-media thickness (CIMT) was measured by ultrasound. The study showed that thicker CIMT in young adulthood is associated with ETS in pregnancy, especially paternal smoking35). However, longitudinal studies are necessary to determine the potential causal relevance of these associations.
Hypertension is the leading risk factor for cardiovascular disease36). Newborn infants of smoking parents show symptoms of cardiovascular stress hyperreactivity. Maternal smoking leads to long-lasting 'reprogramming' of infant blood pressure control mechanisms. The underlying dysfunction in a smoker's infant could plausibly be a precursor or an early marker of long-term susceptibility to complications, such as increased blood pressure37).
Simonetti et al.36) found that both systolic (+1.0 mmHg) and diastolic (+0.5 mmHg) blood pressure were higher in children of smoking parents. In healthy preschool children, parental smoking is an independent risk factor for higher blood pressure, adding to other familial and environmental risk factors36). However, blood pressure in children is not influenced by intrauterine effects38). Because of the high prevalence of risk factors in childhood, primary prevention of cardiovascular diseases should start as soon as children start school39).
ETS and neurobehavioral health in children
Active maternal smoking during pregnancy has been associated with a higher risk of behavioral disorders in children. These disorders range from personality temperament, neuropsychiatric outcomes such as attention disorders (e.g., attention deficit hyperactivity disorder [ADHD]) or conduct disorder, to lowered cognitive abilities40). Maternal smoking habits were associated with lowered cognitive development of children at age 4 years41). The possible explanation for this association is that biological pathways for tobacco neurotoxicity and psychosocial characteristics such as parental education level, intelligence, and mental health may also be involved in the interrelationship between smoking and neurodevelopment. However, results from cohort studies regarding the postnatal effects of tobacco smoke on neurodevelopment are not conclusive.
Data from adoptive families suggest that exposure to parental smoking represents an environmental risk for substance use in the adolescent offspring. In biologically related families, the effect of exposure to parental smoking is larger and more diverse, including substance use, disruptive behavior disorders, delinquency, deviant peer affiliations, aggressive attitudes, and preference for risk taking42).
ETS is also associated with an increased risk of psychiatric morbidity. In a population-based study in Finland (n=175,869), 24.7% had psychiatric diagnoses among children of mothers who smoked more than 10 cigarettes a day (OR, 1.85 [95% confidence interval, 1.74 to 1.96] and 13.7% among unexposed children43). Paternal prenatal smoking seems to be associated with conduct/externalizing problems through a causal intrauterine mechanism44).
Several studies also found an association between behavioral problems and maternal smoking45). A recent meta-analysis found that nicotine studies indicated a greater risk of ADHD-related disorders among children whose mothers smoked during pregnancy46).
However, further studies are necessary to reach a conclusion, owing to inconsistencies in these studies.
ETS and smoking biomarkers in children
A valid estimation of the risks associated with tobacco exposure depends on accurate measurement. Nicotine and its major metabolite, cotinine, are commonly used as smoking biomarkers, and their levels can be determined in various biological specimens such as blood, saliva, and urine.
Nicotine is both the primary addictive component of tobacco smoke and a potential toxin47). It is a major constituent of cigarettes, and is highly specific to tobacco smoke48). It has a half-life of approximately 2 to 3 hours in the blood, and is excreted in urine49). About 80% of nicotine is transformed to cotinine in the C-oxidation pathway50).
Cotinine is the major proximate metabolite of nicotine51). Plasma cotinine level correlates better than self-report to various measures of biological effects of smoking52). Cotinine levels in blood can be accurately estimated by measuring cotinine in saliva or urine51). Cotinine in different body fluids (plasma, urine, and saliva) has a longer half-life (15 to 19 hours) than that in blood51).
The degree of variability in the conversion of nicotine to cotinine is not great, and, even with this source of imprecision, cotinine levels accurately reflect exposure to nicotine from ETS. Urine cotinine depends on renal function, flow rate, and urinary pH. However, cotinine collection and analysis from these sources has several limitations with relative unreliability.
Hair analysis is a convenient, noninvasive technique for detecting the presence of nicotine exposure. Reduced inter-individual variability in hair makes it easier to standardize measurements. Hair collection does not necessitate special handling and storage measures like those required with body fluid samples53). Because cotinine accumulates in hair during hair growth, it is a unique measure of long-term, cumulative exposure to tobacco smoke4). In a study comparing urine cotinine levels with hair nicotine levels to measure ETS in 94 children aged 1 to 3 years from Norway, the authors found that nicotine in children's hair correlated more closely with parental smoking history (r=0.64, P<0.0001) than did cotinine in urine (r=0.50, P<0.0001)54).
A significant reduction in hair nicotine in subjects who used bleaching or hair dying was observed by Jurado et al.55). Hair cotinine correlates well with other measures of nicotine exposure. In a recent meta-analysis, Florescu et al.56) identified cutoff values for validation of cotinine as a marker for ETS exposure.
The International Agency for Research on Cancer concluded that ETS causes lung cancer in humans. Since tobacco-specific N-nitrosamines are found only in tobacco products or related nicotine-containing materials, their adducts or metabolites should be specific biomarkers of tobacco exposure57). As known carcinogen-derived biomarkers of exposure to ETS, nicotine-derived nitrosoamines, such as 4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanone (NNK), are specific for tobacco exposure and are metabolized to a butanol metabolite, (4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanol (NNAL), and its glucuronide (NNAL-GLUC)57,58). Urine levels of NNAL+NNAL-GLUC are elevated in nonsmokers exposed to ETS. These biomarkers have a more direct relation to cancer risk than cotinine because NNK, but not nicotine, is carcinogenic57).
Several analytical procedures have been developed to quantify nicotine and cotinine in hair and other materials. The 4 broad techniques are colorimetry, chromatography, radioimmunoassay, and enzyme-linked immunosorbent assay. Colorimetry is the least desirable method because of its lack of specificity. Ryu et al.59) recently developed a method based on the highly sensitive liquid chromatography-tandem mass spectrometry technique that requires as little as 1 mg of hair to simultaneously measure nicotine and cotinine.
A meta-analysis reviewed the reference values for hair cotinine as a biomarker of ETS. Among unexposed nonsmokers, mean hair cotinine was 0.3 to 0.4 ng/mg in children. A cutoff value of 0.2 ng/mg was accurate in discriminating between exposed and unexposed children56).
ETS and health policy
From the various investigations carried out worldwide on the effects of smoking bans on ETS, it is clear that the policies result in considerable reductions in environmental smoking exposure. Several studies were performed to evaluate the impact of the smoke-free air policy indoors and outdoors. The US Center for Disease Control and Prevention carried out investigations on the level of serum cotinine in nonsmokers during the past decade, and found a reduction of approximately 70%, comparing pre- and post-ban levels60). Pearson et al.61) showed that the smoke-free indoor air policy was effective, dramatically reducing cotinine levels and almost eliminating reports of sensory symptoms due to ETS.
However, the smoking ban policy is not effective in protecting children and young adults from ETS exposure, because their primary source of ETS is typically the home. In Korea, we found that neonatal nicotine concentrations were significantly higher in indoor smokers than in outdoor smokers and nonsmokers3). These findings indicate that paternal smoking inside the home leads to significant fetal and maternal exposure to ETS and may subsequently affect fetal health (Fig. 1).
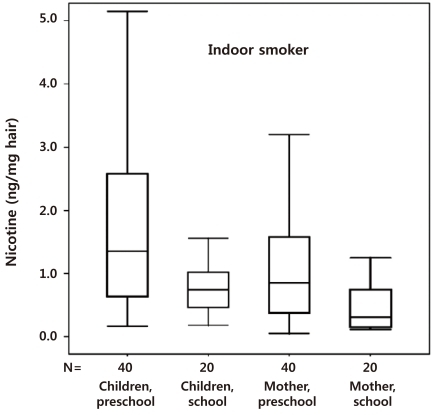
We also reported that the hair nicotine level of women whose spouses only smoked outside the home was still significantly higher than the level of those with nonsmoking spouses62). Thus, ETS is not completely prevented by smoking outdoors. Preschool children are more susceptible to secondhand smoke exposure than nonsmoking mothers and school children (Fig. 2). Thus, a strategy based on the separation of preschool children and pregnant women from the smoking activity of spouses might be inadequate to protect preschool children from ETS at home63), and public policies to reduce ETS exposure should be revised.
Conclusion
ETS exposure significantly contributes to morbidity and mortality in children. Children, in particular, seem to be the most susceptible population to the harmful effects of ETS. Sufficient evidence indicates a significant association between ETS and health problems in children, including respiratory diseases, infection, and neurobehavioral problems. Pediatricians need to be concerned about these adverse effects of ETS exposure.
Children are exposed to tobacco smoke not only in their homes but also in schools, restaurants, child-care settings, cars, buses, and other public places in many countries, including Korea where smoking is culturally and socially allowable. More effective strategies and public policies to protect preschool children from ETS should be consolidated.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


