Article Contents
| Clin Exp Pediatr > Epub ahead of print |
|
Abstract
Background
The effect of vitamin E supplementation on bilirubin levels in infants was previously explored, but the results were inconclusive.
Purpose
To examine the effect of vitamin E supplementation on bilirubin levels in term infants in the neonatal intensive care unit (NICU).
Methods
This interventional double-blind randomized clinical trial was conducted in the Sanandaj Besat Hospital NICU. Enrolled newborns were between 37 and 42 weeks and 6 days of gestation and required phototherapy according to American Academy of Pediatrics clinical guidelines. A total of 138 infants were randomly assigned to vitamin E (n=68) or placebo (n=70) groups. In addition to phototherapy, the vitamin E group received 0.5 mL (5 IU) of supplemental vitamin E daily, whereas the placebo group received 0.5 mL of oral dextrose daily. STATA 17 was used for the data analysis.
Results
Changes in bilirubin levels at 24 hours postintervention did not differ significantly from baseline in either group. Vitamin E supplementation did not significantly reduce total bilirubin levels at 24 hours postintervention (mean difference [MD], -0.18; P=0.204; 95% confidence interval [CI], -1.39 to 1.02). However, the vitamin E group exhibited lower total bilirubin levels than the placebo group at 48 hours postintervention (MD, 0.18; P=0.365; 95% CI, -0.89 to 1.27) and 72 hours (MD, 0.36; P=0.356; 95% CI, -2.34 to 1.61), although the differences were not statistically significant. A subgroup analysis revealed that female infants experienced a greater reduction in total bilirubin levels than male infants.
Conclusion
Infants administered vitamin E versus placebo demonstrated similar reductions in bilirubin levels and hospital stays. Although the average bilirubin changes did not differ significantly between groups, the vitamin E group showed a more noticeable reduction over time, indicating a positive effect of vitamin E supplementation on serum bilirubin reduction.
The most common cause of hospitalization during the neonatal period is neonatal hyperbilirubinemia, which is defined as a total serum bilirubin (TSB) level greater than 5 milligrams/deciliter [1,2]. Approximately 50% of term infants and 80% of preterm infants suffer from this disorder during the first week of life. Furthermore, this condition is one of the main causes of readmission after early discharge in infants [3]. Neonatal hyperbilirubinemia occurs in infants due to various factors, such as increased bilirubin production, enhanced enterohepatic circulation, reduced clearance of unconjugated bilirubin, and metabolic disorders like hypothyroidism. Conditions such as hemolysis, cephalohematoma, polycythemia, sepsis, and macrosomia can lead to excessive bilirubin production in neonates. Lipid autoxidation on the surface of red blood cells is considered one mechanism that may contribute to increased bilirubin production by inducing hemolysis [1,2,4-6]. Unconjugated bilirubin can pass through the blood-brain barrier due to various reasons, and lead to incidence of severe neurological complications such as kernicterus [6,7].
According to the American Academy of Pediatrics (AAP), the TSB level is the strongest predictor of kernicterus risk in term babies with jaundice. Excessive levels of 25 mg/dL can cause kernicterus, and the AAP recommends strong measures at this level [8,9]. The main strategies for treating hyperbilirubinemia include phototherapy and blood transfusion. Phototherapy is the initial treatment and is used in most preterm infants as a preventive measure to avoid further increases in TSB [10]. Phototherapy side effects consist of skin rash, retinal damage and slow weight gain [11].
Alpha-tocopherol, commonly known as vitamin E, is a form of antioxidant that can reduce the progression of several diseases in premature babies by scavenging free radicals [12]. It is essential for protecting cells from oxidative stress, regulating immunological function, and maintaining endothelial cell integrity [13]. A variety of cells in the body are able to absorb vitamin E, which is then incorporated into the endoplasmic reticulum for its antioxidant function [13]. This prevents reactive oxygen radicals from changing or deactivating intracellular pathways and molecules [13]. Vitamin E may protect cellular structures from free radical attack and protect red blood cells against hemolysis [12]. Vitamin E also prevents and stabilizes phospholipid peroxidation of erythrocyte membranes, thereby reducing erythrocyte lysis and hemoglobin synthesis in the blood, which can lead to lower serum bilirubin levels [14].
The relationship between the administration of antioxidants, such as vitamin E, and TSB levels and length of hospital stay in infants with hyperbilirubinemia has been the subject of several studies [3,15-19]. Some studies showed that the use of vitamin E can significantly reduce TSB levels and the duration of phototherapy [15,16]. However, another trials found no significant association between vitamin E supplementation and a reduction in the duration of phototherapy in infants [3,18,19], so the relationship between vitamin E and TSB levels in infants and the duration of phototherapy is inconclusive. The present study was designed to evaluate the effect of vitamin E on hyperbilirubinemia in term infants, given the limited number of studies in this area.
This study was a double-blind randomized clinical trial conducted on 138 term neonates diagnosed with hyperbilirubinemia in the neonatal intensive care unit (NICU) of the Besat Hospital in Sanandaj, Iran, from the beginning of September 2022 to the end of January 2023.
After obtaining ethical approval from the Kurdistan University of Medical Sciences Ethics Committee and receiving the ethics code and receiving the clinical trial code in the registration system of clinical trial study protocols in Iran (https://www.irct.ir), the study was initiated. The Pediatrics Department of the Medical School faculty members assessed the conformity of the study protocol with clinical and scientific standards. The protocol received approval from the University Ethics Committee on March 12th, 2022, with the code IR.MUK.REC.1400.326, before being implemented. Moreover, the protocol was registered in the Iranian Registry of Clinical Trials (IRCT) on August 26th, 2022, with the identifier IRCT20220806055625N2.
This study focused on newborns born at term (with gestational age of 37 to 42 weeks and 6 days) and weighing between 2,500 and 4,000 g who were diagnosed with hyperbilirubinemia who met 2 criteria: (1) the manifestation of jaundice occurring after 24 hours from birth; (2) a TSB level surpassing the 40th percentile indicated by the age-specific bilirubin nomogram provided by AAP. 20) The exclusion criteria included any clinical or laboratory evidence of infection, any congenital abnormalities, maternal history of phenobarbital use, hypothyroidism, intrauterine growth restriction, dehydration, feeding intolerance, and mechanical ventilation. A set of tests, including maternal and neonatal blood type determination, hemoglobin, complete blood count, reticulocyte count, peripheral blood smear, Coombs test, thyroid function test, and G6PD (glucose-6-phosphate dehydrogenase) test were performed to rule out the exclusion criteria.
The sample size for this study was determined using the findings of a previous study conducted by El Mashad et al. [15], with a power of 80% and a confidence interval of 95%. STATA 17 (StataCorp LLC, College Station, TX, USA) was used to perform the calculation. Ultimately, a sample size of 30 individuals was estimated for each group. To account for potential sample dropout and increase the study's power, the sample size in each group was increased up to a maximum of 70 individuals.
After selecting the newborns, their legal guardians were informed about the benefits and risks of the intervention, and written consent was obtained from them. It was explained to them that all newborns would receive the usual treatment for hyperbilirubinemia (phototherapy), but in rare cases where the intervention (vitamin E) caused vomiting, diarrhea, or drug hypersensitivity, the drug would be immediately discontinued, and appropriate treatments, including blood transfusion, would be administered if necessary. The randomization process was performed using simple randomization with Microsoft Excel software (using the "Ran between" command). Initially, each selected newborn was assigned a unique three-digit code for the randomization process. Then, randomization was performed using these codes, and 70 codes were assigned to the phototherapy group with vitamin E, while the next 70 codes were assigned to the phototherapy group with a placebo. The researcher responsible for drug prescription and evaluation was not involved in the allocation process. Moreover, the researcher responsible for the analysis was not informed about the group assignments.
All infants were treated with intensive phototherapy according to the clinical practice guidelines of the AAP [21]. Phototherapy was administered to both groups using a Tusan device equipped with 4 Philips lamps, positioned 25 centimeters away from the newborn's surface, with a minimum radiation intensity of 10 ╬╝W/cm2/nm.In addition to phototherapy, the intervention group received oral vitamin E at a dose of 0.5 mL (equivalent to 5 IU; BEHSA Company, Tehran, Iran) once a day [22], while the placebo group received 0.5 mL of 10% dextrose serum orally daily. The placebo was identical to vitamin E in terms of volume, color, appearance, and packaging. Oral droppers were used for administration.
The vitamin E syrups and placebos were kept in bottles with similar shapes and colors, labeled as A and B, and were prescribed by the researcher and administered to the newborns by nurses. The syrups were administered at the beginning of hospitalization and then every 24 hours until the newborns no longer needed phototherapy (i.e., when their TSB level reached below the threshold for phototherapy of less than 2 mg/dL) [23].
The level of bilirubin was assessed using blood samples from neonates and their medical records as the study's outcome. Nurses collected blood samples and sent them to the laboratory for analysis. All nurses participating in medication administration and blood sample collection, as well as laboratory personnel, were blinded to the type of medicationusedinthe2groups.Tocollectinformationabout the infants, including the type of medication administered and their bilirubin levels, one of the researchers, who was the only person aware of the treatment and its effect, completed a checklist.
Bilirubin levels were assessed before and after phototherapy at 24, 48, and 72 hours. Direct, indirect, and total bilirubin levels were assessed using the Diazo method of Pearlman at the reference laboratory of the university, utilizing the BT-3000 auto analyzer.
The number of hours of phototherapy received and the number of days hospitalized were also among the desired outcomes. According to the guidelines, total hours of phototherapy were defined as the total time a neonate was under radiation in the Tusan device. Furthermore, in this study, the number of days hospitalized was collected from the medical records of newborns, which were documented by ward nurses at the time of discharge from the hospital.
The data was analyzed using STATA 17 software. The Kolmogorov-Smirnov test was used to check for normality, while descriptive tests such as mean and standard deviation were used for quantitative variables and frequency and count were used for qualitative variables. To compare the outcomes of interest between the 2 groups at different time points, repeated measures analysis of variance (ANOVA) and other appropriate tests such as independent or paired t tests were utilized. A significance level of 0.05 was considered for this study.
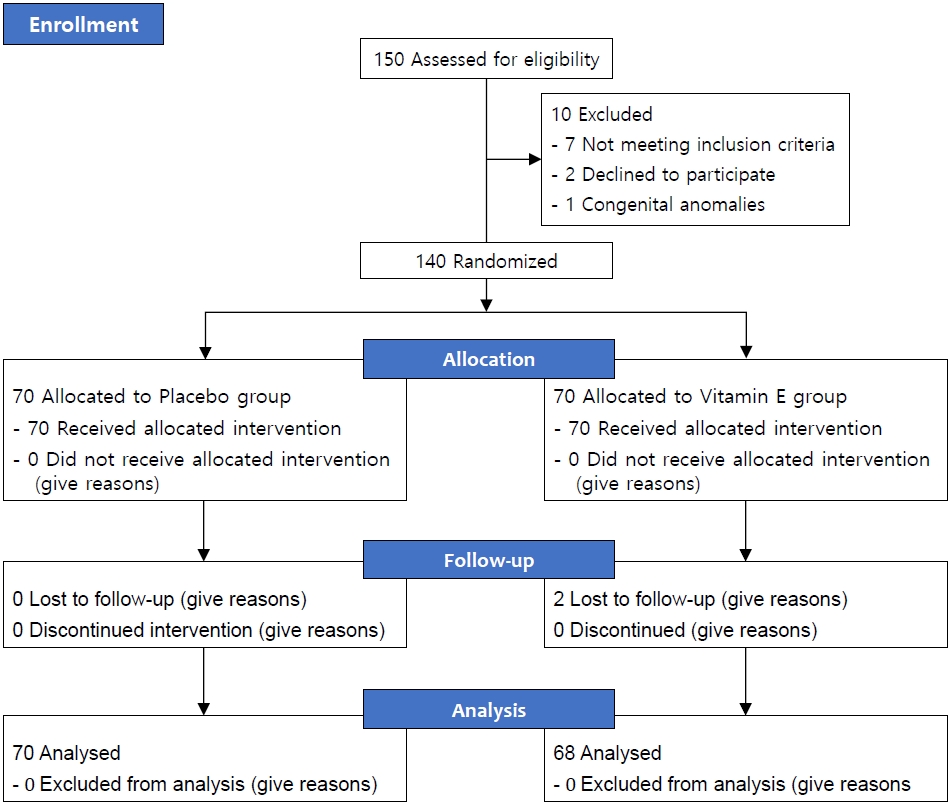
During the study period of 5 months, 150 newborns were identified as potential candidates for admission to the NICU at Besat Hospital in Sanandaj. However, 8 of these newborns did not meet the inclusion criteria, 2 guardians of newborns did not agree to participate, and 2 other newborns were excluded due to congenital anomalies. Therefore, 138 newborns were randomly divided into 2 groups: one group received phototherapy with vitamin E drops (n=68), and the other group received phototherapy with a placebo (n=70) (Fig. 1).
The baseline characteristics and other factors that could affect TSB were distributed evenly between the intervention group and the placebo group (Table 1). The age at entry into the study (4.8┬▒3.0 days vs. 3.5┬▒1.5 days) was similar in both groups. The TSB at inclusion (16.65┬▒3.13 mg/dL vs. 16.37┬▒3.33 mg/dL) was similar in 2 groups. According to the results, there was no significant difference between the 2 groups in terms of gestational age (38.10┬▒1.02 g vs. 38.46┬▒1.24 g), birth weight (3,036.14┬▒425.35 g vs. 3,002.35 ┬▒399.28 g), direct bilirubin (0.66┬▒0.22 mg/dL vs. 0.69┬▒0.21 mg/dL), mean 72 hours of receiving phototherapy (11.11┬▒ 1.75 vs. 11.31┬▒1.51), and length of hospital stay (2.60┬▒0.78 days vs. 2.58┬▒0.67 days)(Tables 1, 2).
To compare the mean changes in TSB levels from baseline to 24, 48, and 72 hours after the intervention, repeated measures ANOVA was used. The results showed no significant difference in the mean changes in TSB levels between the 2 groups at 24 hours (MD, -0.18; P=0.204; 95% CI, -1.39 to 1.02), 48 hours (MD, 0.18; P=0.365; 95% CI, -0.89 to 1.27), and 72 hours (MD, 0.36; P=0.356; 95% CI, -2.34 to 1.61) after the intervention. However, in both groups, there was a significant and considerable decreasing trend in TSB levels over time (Table 3). One notable finding of this study was that the average TSB levels in the group receiving vitamin E decreased more than the placebo group after 72 hours of the intervention (MD, -12.01; P<0.001; 95% CI, -15.52 to -8.50) (Table 3).
In this study, the mean direct bilirubin levels were also examined and compared between the 2 groups, and the results are presented in Table 3. The findings revealed that the mean changes in direct bilirubin levels from baseline to 24 and 48 hours after the intervention were significantly greater in the intervention group, and the reduction in direct bilirubin levels was greater in the vitamin E group. However, there was no significant difference between the 2 groups in terms of mean changes in direct bilirubin levels from baseline to 72 hours after the intervention. In terms of mean differences between the 2 groups relative to baseline, at 24 hours after the intervention, the mean direct bilirubin level in the placebo group decreased by 0.06 (P=0.07), while in the vitamin E group, it not only did not decrease but increased by 0.02 (P=0.17). At 48 hours after the intervention, the mean direct bilirubin level in the vitamin E group decreased by 0.05 relative to baseline (P=0.11), and this reduction reached 0.17 at 72 hours after the intervention (P=0.08) (Table 3).
The changes in TSB levels among males and females are presented in Table 4. The findings showed that during the first 24 hours, there was a significant reduction in bilirubin levels in males in both the intervention and placebo groups. The same trend was observed after 48 hours in both male and female, but there was no significant difference between them. However, after 72 hours, the reduction in TSB levels was significantly higher in females in both groups (Table 4). No adverse effects of therapy by vitamin E were reported.
This clinical trial was conducted to investigate the effect of vitamin E on serum bilirubin levels, duration of phototherapy, and length of hospitalization in term infants admitted to the NICU of Besat Hospital in Sanandaj, Kurdistan province,Iran.
The results showed a significant reduction in TSB levels in both groups during the treatment period, with a decrease also observed in the placebo group. However, it is worth noting that the reduction in TSB levels was more significant in the group receiving vitamin E compared to the placebo group at different time points. Therefore, it can be inferred that the effect of vitamin E on bilirubin reduction is more prominent and remarkable as time passes.
This conclusion is consistent with results from previous studies conducted globally. For instance, Fischer et al. [19] demonstrated that vitamin E supplementation had no significant effect on bilirubin levels in the first 3 days of life, especially in premature infants. However, after that period, a notable decrease in bilirubin levels was observed, which could suggest a delayed effect of vitamin E on bilirubin levels. In another study, Barekatain et al. [16] found that the reducing effect of vitamin E on bilirubin levels in preterm infants may be seen after 3 days of intervention, suggesting a delayed effect similar to the current study on hyperbilirubinemia. In addition, Barekatain B et al. [16] showed that the impact of vitamin E consumption on reducing bilirubin levels in infants increases notably as the infants get older. Bilirubin is a yellow pigment in the blood that forms as a result of the breakdown of hemoglobin [24]. Hyperbilirubinemia occurs when the level of bilirubin in the blood exceeds normal levels [25]. Vitamin E is a powerful antioxidant that can prevent oxidative damage to cells and improve overall health [26]. However, it can be argued that vitamin E consumption alone cannot directly affect bilirubin levels in the blood and cannot be considered a treatment for high bilirubin levels. More clinical trials with larger sample sizes are required to confirm this hypothesis. Therefore, currently the best way to reduce high bilirubin levels is to accurately diagnose the underlying cause.
The results of the clinical trial conducted by El Mashad suggest that using vitamin E alone may not be effective in reducing bilirubin levels [15]. Therefore, it is recommended to use vitamin E in combination with phototherapy to achieve a more meaningful and significant effect. During the present study, both phototherapy and vitamin E were administered together. Although vitamin E did have a notable effect on reducing TSB levels, there was no significant difference observed between the 2 groups.
According to the study,there was no significant difference observed in the number of hours of phototherapy received between the 2 groups. This lack of difference may have been caused by the mothers' preferences for putting their babies in the phototherapy device and their spontaneous tendency to exit the infants from phototherapy device for feeding or other reasons. As a result, there may have been a loss of the phototherapy's potential confounding effect on the drug's efficacy in altering serum bilirubin levels. In the study conducted by Gross [27], 20 infants with birth weight between 1,000 and 1,500 g and 20 infants with birth weight between 1,500 and 2,000 g were examined. Half of the infants in each group received vitamin E intramuscularly at a total dose of 50 mg/kg daily for the first 3 days of life. The duration of phototherapy in the group receiving vitamin E was significantly shorter. This difference in phototherapy duration could be due to better cooperation of participating mothers, differences in drug administration, or differences in the study population, which had lower birth weight and fewer infants.
The study found no significant difference in the number of hospitalization days between the 2 groups. One of the reasons for this could be the early discharge of infants from the study by parental consent without medical order.In line with the present study's findings, in the study conducted by El Mashad et al. [15] on 150 infants with hyperbilirubinemia, no significant difference was observed in hospitalization duration between the group receiving vitamin E (at doses of 25 mg/kg/day and 50 mg/kg/day orally) and the group receiving phototherapy alone. Al-Banna et al. [3] also conducted a study on the effects of prescribing antioxidant vitamins in the treatment of neonatal hyperbilirubinemia. The results were consistent with the findings of the current clinical trial, showing no significant difference in the number of hospitalization days between the intervention and control groups (P=0.06). Similar to the present study, the infants enrolled in this study were all term infants, but they had a birth weight of less than 2000 g. Fischer and colleagues conducted a randomized double-blind study on the efficacy of oral vitamin E supplementation as a preventive treatment for hyperbilirubinemia in preterm infants weighing less than 1,500 g [19]. The TSB levels were measured in each infant on the first and third days of the study. Similar to the present study, no significant difference was found between the treatment group receiving vitamin E and the control group receiving placebo in terms of serum bilirubin levels on days 1 or 3. Stevenson et al. [18] observed that in a group of preterm infants undergoing treatment with vitamin E (5 mg/kg intra-arterial infusion), the serum bilirubin levels on days 2, 3, and 4 did not differ between the vitamin E group and the control group receiving placebo, which is consistent with our findings.
This study was the first randomized double-blind clinical trial examining the effect of vitamin E supplement on hyperbilirubinemia in term infants. The results indicated that there was no significant difference between the 2 groups in terms of serum bilirubin levels, duration of phototherapy, and length of hospital stay. These findings were contrary to the results of Ghada's study, which concluded that administering vitamin E supplements at a dose of 25 mg/kg/day could significantly reduce TSB levels on the second and third days [15]. The current study aimed to conduct a more detailed examination by enlarging the sample size and utilizing a placebo instead of a control group to compare the drug's efficacy. The aim was to conduct accurate randomization of the 2 groups, which would support the fact that there was no significant difference between the 2 groups in terms of TSB levels at admission.
One of the study limitations was not considering maternal breastfeeding practices (breast feeding or formula). Another limitation was the discharge of infants less than 72 hours after the intervention due to the absence of clinical indications for continued hospitalization and parental intolerance. If a larger sample of infants had been administered the drug for 72 hours and monitored for bilirubin levels in this study, it would have been possible to observe the complete and significant effect of vitaminEon bilirubin. However, due to scientific and ethical considerations, it was not possible to extend the infants' hospital stay for a longer period of time in this study. Additionally, the limited sample size was due to the fact that only a small number of studies have been conducted on this issue so far, and they have included small sample sizes. Therefore, information about the study design and the participating population was limited.
In conclusion, the study suggests that short-term use of vitamin E supplement can potentially reduce bilirubin levels in term infants with hyperbilirubinemia, but the observed effect was comparable to placebo in this study due to limitations such as small sample size and short follow-up period. To evaluate the effectiveness of vitamin E on hyperbilirubinemia more precisely, a larger sample size and better study designs considering longer follow-up periods are required. Once more accurate results are available, a more informed decision can be made regarding the use of vitamin E for reducing bilirubin levels in term infants with hyperbilirubinemia.
Footnotes
Acknowledgments
The authors would like to thank the healthcare staff of Besat Hospital for providing information in response to inquiries and providing assistance in data processing.
Table┬Ā1.
Baseline characteristics of patients in placebo versus vitamin E groups
Table┬Ā2.
Phototherapy duration and admission time by study group
Table┬Ā3.
Mean difference (MD) between baseline and total and direct bilirubin levels by study group
Table┬Ā4.
Mean difference (mg/dL) between baseline and total bilirubin levels by study group and sex
References
1. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician 2014;89:873ŌĆō8.

3. Al-Banna SM, Riad AN, Anis SS. The effect of fenofibrate and antioxidant vitamins [D, E and C] in treatment of uncomplicated neonatal hyperbilirubinemia. Ann Neonatol J 2020;2:37ŌĆō48.

4. Rathore S, Kumar Vk C, R S. A critical review on neonatal hyperbilirubinemia-an Ayurvedic perspective. J Ayurveda Integr Med 2020;11:190ŌĆō6.


5. Abdul-Razzak KK, Nusier MK, Obediat AD, Salim AM. Antioxidant vitamins and hyperbilirubinemia in neonates. Ger Med Sci 2007;5:Doc03.


6. Pace EJ, Brown CM, DeGeorge KC. Neonatal hyperbilirubinemia: an evidence-based approach. J Fam Pract 2019;68:E4ŌĆō11.

7. Bhutani VK, Johnson L. Kernicterus in the 21st century: frequently asked questions. J Perinatol 2009;29 Suppl 1:S20ŌĆō4.



8. Wennberg RP, Ahlfors CE, Bhutani VK, Johnson LH, Shapiro SM. Toward understanding kernicterus: a challenge to improve the management of jaundiced newborns. Pediatrics 2006;117:474ŌĆō85.



9. Ip S, Chung M, Kulig J, O'Brien R, Sege R, Glicken S, et al. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics 2004;114:e130ŌĆō53.



10. Bhutani VK, Committee on Fetus and Newborn; American Academy of Pediatrics. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2011;128:e1046ŌĆō52.



11. Orzalesi M. Advantages and disadvantages of phototherapy (PT) in neonatal hyperbilirubinemia. In: Castellani A, editor. Research in photobiology. Boston (MA): Springer, 1977:419ŌĆō29.

12. Gonz├Īlez-P├®rez O, Moy-L├│pez NA, Guzm├Īn-Mu├▒iz J. El alfatocoferol y el acido alfa-lipoico. Una sinergia antioxidante con potencial en medicina preventiva Alpha-tocopherol and alpha-lipoic acid. An antioxidant synergy with potential for preventive medicine. Rev Invest Clin 2008;60:58ŌĆō67.

13. Vitamin E. LiverTox: clinical and research information on drug-induced liver injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2012.
14. Anosike CA, Igboegwu ON, Nwodo OFC. Antioxidant properties and membrane stabilization effects of methanol extract of Mucuna pruriens leaves on normal and sickle erythrocytes. J Tradit Complement Med 2018;9:278ŌĆō84.



15. El Mashad GM, El Sayed HM, El NAAES. Role of vitamin E supplementation in neonates with hyperbiirubinemia. Menoufia Med J 2019;32:734.

16. Barekatain B, Sadeghnia A, Moradi N, Yazdi M. Effects of Vitamin E on Neonatal Hyperbilirubinemia in Preterm Newborns. Adv Biomed Res 2022;11:86.



17. Rohana J, Mahdy Z, Siraj H, Dali A, Khazaai H, Mutalib M. The role of antenatal vitamin E supplementation in the prevention of neonatal jaundice. JNPM 2010;3:217ŌĆō21.

18. Stevenson DK, Vreman HJ, Ferguson JE 2nd, Lenert LA, Leonard MB, Gale R. Continuous parenteral infusion of vitamin E pharmacokinetics and bilirubin production in premature neonates. Ann N Y Acad Sci 1989;570:352ŌĆō7.

19. Fischer AF, Inguillo D, Martin DM, Ochikubo CG, Vreman HJ, Stevenson DK. Carboxyhemoglobin concentration as an index of bilirubin production in neonates with birth weights less than 1,500 grams: a randomized double-blind comparison of supplemental oral vitamin E and placebo. J Pediatr Gastroenterol Nutr 1987;6:748ŌĆō51.


20. Kemper AR, Newman TB, Slaughter JL, Maisels MJ, Watchko JF, Downs SM, et al. Clinical practice guideline revision: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2022;150:e2022058859.

21. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297ŌĆō316.



22. Granati B, Largajolli G, Rubaltelli FF, Pollazzon P, Bottos M, Sartori E. Efficacy and safety of the "integral" phototherapy for neonatal hyperbilirubinemia. Results of a follow-up at 6 years of age. Clin Pediatr (Phila) 1984;23:483ŌĆō6.



23. Bansal A, Jain S, Parmar VR, Chawla D. Bilirubin rebound after intensive phototherapy for neonatal jaundice. Indian Pediatr 2010;47:607ŌĆō9.



24. Kalakonda A, Jenkins BA, John S. Physiology, bilirubin. In: StatPearls [Internet].Treasure Island (FL): StatPearls Publishing; 2024 Jan-.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


