Pneumococcal conjugate vaccines: choice of schedule and product development
Article information
It is widely known that the successful use of pneumococcal conjugate vaccines (PCVs) has resulted in a marked reduction in invasive pneumococcal diseases in children. However, the World Health Organization estimates that Streptococcus pneumonia still kills close to half a million children under 5 years old worldwide every year; most of these deaths occur in developing countries [1]. By the end of 2018, only 47% of 1-year-olds worldwide had received 3 doses of PCV. This measure serves as an indicator of health system performance in efforts to eradicate diseases. Such children have not been vaccinated because their country has not introduced the vaccine or they are not covered by its National Immunization Program (NIP) [2]. As of March 2019, 144 countries (74%) have introduced PCV into their NIP, 15 (8%) have announced plans to do so, and 35 (18%) have yet to make a decision [2]. Thus, further increasing PCV coverage globally is required, which appears to be closely related to the economic situation of each country.
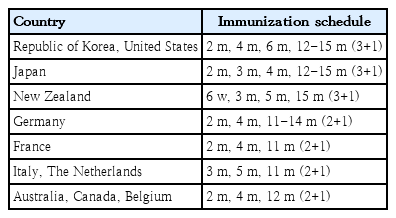
Two kinds of PCV (10-valent Synflorix and 13-valent Prevnar 13) are currently available worldwide with 4 approved dosing regimens (3 primary doses with 1 booster [3p+1] schedule). The NIP of countries such as the United States and the Republic of Korea (23 countries as of March 2019) use a 3p+1 schedule [3]. The seven-valent Prevenar was first used in 2003 in the Republic of Korea. The PCV10 (Synflorix) and PCV13 (Prevnar 13) vaccinations were introduced in 2010, and NIP for children with PCV10 or PCV13 were first implemented in 2014. Alternatively, it can be administered as a 3-dose schedule as 3 primary doses without a booster (3p+0 schedule) or as 2 primary doses with 1 booster (2p+1 schedule). The 3p+0 schedule is being adopted in 61 countries, while the 2p+1 schedule is being adopted in 59 countries (Fig. 1, Table 1). The 3-dose schedule is also known as a dose-sparing schedule. The 2p+1 schedule has potential benefits over the 3p+0 schedule since higher antibody titers are induced in the second year of life [3]. Three- and 4-dose schedules have been proven effective with both direct and indirect effects against pneumococcal disease caused by vaccine serotypes [4,5].

Pneumococcal conjugate vaccine – current dosing schedule by country. Source: International Vaccines Access Center (exported from www.VIEW-hub.org).
GBP411, a 12-valent PCV (serotypes 1, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F), was developed to enable practical dosing that meets the needs of low-income countries with a low vaccine introduction rate and a higher mortality rate than those of high-income countries [1]. Unlike PCV13, GBP411 uses CRM197 as a carrier protein and polysorbates as suspending agents. The investigators assessed the immunogenicity of GBP411 compared to PCV13 in a phase 2 trial [6]. A total of 3 doses of the GBP411 or control vaccine were administered to subjects on the 2p+1 schedule. This study demonstrated that after the booster dose, >97% of the subjects achieved an immunoglobulin G (IgG) concentration ≥0.35 μg/mL for all 12 serotypes. After the primary doses, for a few serotypes (6B and 19A), the proportion of subjects who met the immunogenicity criteria was significantly lower in the GBP411 group than in the control vaccine group. Low immunogenicity for serotypes 6B and 23F with a 2-dose primary schedule is known [7]. However, it is unknown whether a lower serotype-specific geometric mean concentration (GMC) of an antibody indicates lower efficacy for those serotypes [3]. No serotype-specific thresholds for antibody concentrations have been defined to date. Data on local epidemiology based on serotype prevalence should be considered when choosing a schedule. The safety profile of GBP411 was similar to that of the control vaccine.
The occurrence of pneumococcal disease caused by nonvaccine serotypes has increased. A candidate 15-valent PCV (PCV15) has been developed that includes 2 more serotypes (22F and 33F) that are among leading causes of invasive pneumococcal disease following PCV implementation. The PCV15 vaccine was developed for inoculating patients at 2, 4, 6, and 12–15 months of age (3p+1 schedule). A phase 2 study compared the safety and immunogenicity of PCV15 versus PCV13 in infants. Safety profiles were comparable across vaccination groups. At postdose-3, PCV15 formulation was non-inferior to PCV13 for 10 of 13 shared serotypes but inferior for 3 serotypes (6A, 6B, and 19A) based on the proportion of subjects achieving an IgG GMC of 0.35 g/mL and induced higher antibodies to serotypes 3, 22F, and 33F than to PCV13 [8].
Choice of PCV schedule and products remains a complex issue under debate. Many countries use a 3-dose schedule for PCV immunization, and the new 12-valent PCV is expected to be used as a practical vaccine using the 2p+1 schedule. In choosing schedules and products, each country should consider programmatic factors, including timeliness of immunization and coverage. A cost-benefit analysis will also be required.
Notes
No potential conflict of interest relevant to this article was reported.