Article Contents
| Clin Exp Pediatr > Volume 61(6); 2018 |
|
Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease in children. Patients with AD experience a high rate of colonization of the skin surface by Staphylococcus aureus. Because of a skin barrier defect, there is a potential risk of staphylococcal invasive infection in patients with AD. Here, we present 2 cases of breast abscess caused by S. aureus in 2 adolescent girls with severe AD. Methicillin-sensitive S. aureus was identified from the breast abscess material. They were treated with appropriate antibiotics, however surgical drainage of the abscess was needed in case 1. Identical strains were found from the breast abscess material as well as the lesional and the nonlesional skin of the patients through matrixassisted laser desorption/ionization time-of-flight analysis. We characterized the differential abundance of Firmicutes phylum in patients' skin in microbiota analysis. In particular, S. aureus, a member of Firmicutes, differed significantly between the lesional and the normal-appearing skin. Our cases demonstrate the potential severity of bacterial deep tissue infection in AD and the dysbiosis of skin microbiota may be involved in inflammation in AD.
Atopic dermatitis (AD) is a chronic relapsing pruritic skin disease in children. Colonization of Staphylococcus aureus (S. aureus) is commonly observed in the skin lesions of AD patients,1) but rare cases can be complicated with deep infections. The breached skin of most AD patients is heavily colonized with S. aureus. Due to high bacterial density, altered skin barrier, and immune dysregulation, AD can cause normally colonized S. aureus to penetrate deep into the tissue.2) Such patients have increased serine protease activity which leads to a compromised skin barrier and possibly increases S. aureus colonization.3) Thus, high rate of cutaneous colonization by S. aureus in AD represents an important source of invasive bacterial infection. Breast abscess is defined a local accumulation of pus within the breast due to infection. Mastitis or breast abscess is an uncommon disease in childhood; however, it occurs in 2 distinct age groups, neonates and adolescents.4) Breast abscess is a rare but possible complication in childhood AD, and here we report 2 cases with breast abscess in children with AD.
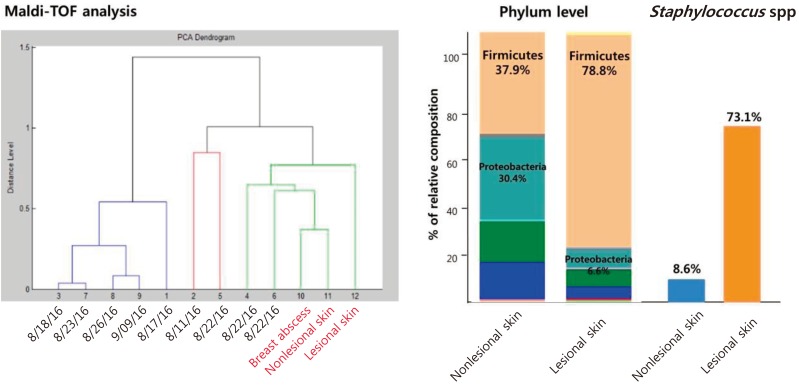
A 13-year-old girl was admitted to Korea University Anam Hospital with pain and swelling of the left breast for 2 weeks. She suffered from AD since age 8 years. She was intermittently treated with systemic steroids and cyclosporine in addition to topical steroid ointment since 2015. She had neither family history of allergic diseases nor other accompanying allergic diseases. Her height was 157 cm, weight 59.4 kg, and body mass index (BMI) 24.0 kg/m2. She had extremely dry skin with lichenification, generalized dermatitis with oozing, desquamation, and numerous scratch marks. Her SCORing of Atopic Dermatitis (SCORAD) index was 56. Scoring of extent, intensity, and subjective symptoms (pruritus and sleep loss) were 36, 10, and 14 (8 and 6), respectively. On physical examination, her left breast was swollen, erythematous and tender (Fig. 1A). White blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and C reactive protein (CRP) were 14,400/µL (neutrophil 74.5%), 29 mm/hr, and 60.1 mg/L, respectively, suggestive of systemic infection. The serum total IgE level was elevated (486.2 IU/L). Specific IgE (sIgE) to cat fur and dog hair were both class 6, and sIgE to Candida albicans was class 2. The serum levels of IgA, IgM, and IgG were all within the normal range. Sonographic examination of the left breast showed a large abscess pocket with adjacent inflammatory changes in the left subareolar area with reactive lymph nodes in the left axilla (Fig. 1B). Methicillin-sensitive S. aureus was identified 3 days after culture of the abscess material. Despite treatment with intravenous cefazolin (first generation cephalosporin) and aspiration of pus material, the breast abscess worsened. Surgical drainage yielded approximately 100 mL of pus. Culture of eczematous and normal-appearing skin was also positive for S. aureus. Identical strains were found from the abscess material and skin culture through Matrix-assisted laser desorption/ionization time-of-flight analysis (Fig. 2A). Using 16S rRNA gene-targeted sequencing, we evaluated taxa with differential abundance in skin. Altered skin microbiota characterized by an elevated level of Firmicutes and a decreased level of Proteobacteria were found in the lesional area of the AD skin. In particular, S. aureus, a member of the Firmicutes, was more frequently isolated from the lesional area of the AD skin than from normal-appearing skin (Fig. 2B). After further administration of intravenous cefazolin, the breast abscess was completely cured. Her SCORAD index was also decreased from 56 to 28 at discharge.
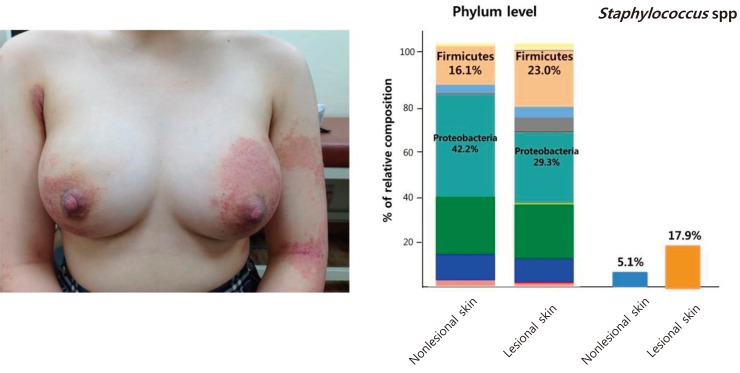
A 15-year-old girl visited our hospital due to exacerbation of pruritus and eczema on the lower back, axillary area and legs for 10 days. She had a history of AD since 7 years of age. She had neither family history nor past history of other allergic diseases. Her height was 160 cm, weight 53.2 kg, and BMI 20.8 kg/m2. Her left breast was swollen, painful, and erythematous (Fig. 3A). She had been treated with topical steroid ointment and emollient, but her eczema worsened 2 months prior to presentation. Her initial SCORAD index was 54. Scoring of extent, intensity, and subjective symptom (pruritus and sleep loss) were 46.3, 11, and 7 (4 and 3), respectively. She had dry skin all over the body and lichenification on the flexor aspects of both elbows and knee joints. Her breast skin was covered with erythematous, oozing, desquamated lesions as well as scratched scars. WBC count, ESR, and CRP were 5,280/µL (neutrophil 63.2%), 2 mm/hr, and 0.17 mg/L, respectively. The serum total IgE level was elevated to 332.0 IU/L and peripheral blood showed 6.3% eosinophils. Serum sIgE to Japanese hop, D. farinae, and cat fur were all class 6. She was diagnosed as having breast abscess through sonographic examinations. Methicillin-sensitive S. aureus was identified 3 days after culture of the abscess material. Identical strains were found from the eczematous and normal-appearing skin. An increased proportion of staphylococcal species was found in skin microbiome analysis (Fig. 3B). Ten days after administration of antibiotics (amoxicillin), her breast abscess was completely cured. Treatment with hydration, a moisturizing emollient, and a topical corticosteroid ointment markedly improved the eczematous skin lesions. Her SCORAD index was also decreased from 54 to 42 after treatment. No recurrence of breast abscess was observed after the treatment in either case.
This report was approved by the Institutional Review Board of Korea University Anam Hospital (approval number: 2018AN0113). Written informed consent was obtained from patients and their parents.
We presented 2 cases of breast abscess in adolescent girls with severe AD that was completely cured by proper antibiotics and surgical drainage. Innate immune system abnormalities, including reductions in antimicrobial peptides, diminished recruitment of cells like neutrophils to the skin, and epidermal barrier defects play a role in S. aureus colonization in the skin of patients with AD.5,6) Although S. aureus colonizes affected and unaffected skin in patients with AD, systemic or serious staphylococcal infections are rare. Only a few cases of septic arthritis, bacteremia, or endocarditis have been reported in the literature.6,7,8,9)
S. aureus is the most commonly found in mastitis or breast abscess. 4) Generally, penicillin or cephalosporin antibiotic is used until drug sensitivity results are retrieved. If the abscess worsens in spite of proper antibiotic use, it may need to be incised and drained. Breast abscess is rare in adolescent girls, primarily occurring in lactating women as well as patients with diabetes mellitus, obesity or immune dysregulation. Tobacco smoking and nipple piercing have been known as risk factors for the development of breast abscess.10) However, in our 2 patients, there are no preceding conditions inducing breast abscess except for severe AD. There was no evidence of congenital immunodeficiency, such as hypo-gamma-globulinemia or Wiskott-Aldrich syndrome. Rather, extreme dryness and pruritus were more likely to be predisposing factors for staphylococcal colonization and infection in our patients.
Intense scratching in AD is known as a predisposing factor for staphylococcal skin and deep tissue infections. It is believed that our patients' scratching of pruritic skin lesions increased the risk for the penetration and spread of S. aureus into the deep tissue. Not surprisingly, identical strains were found from their breast abscesses, involved and noninvolved skins.
S. aureus is isolated from 2%–25% of healthy skin, and it highly colonizes both the lesional and normal-appearing skin of patients with AD.11,12) Once attached to the skin, S. aureus secretes exotoxins with super-antigenic properties that worsen AD.13) A deficiency in the expression of endogenous antimicrobial peptides in the skin of patients with AD, causing localized immunodeficiency, may account for the susceptibility of these patients to skin invasion with S. aureus.11,12) As for humoral immunity, considerable attention has been focused on the importance of the secretory component of immunoglobulin A (sIgA). In a previous study,6) the amount of sIgA secretion from the skin was significantly lower in AD patients than in healthy subjects and the decrease in sIgA accounted for the high positive skin culture of S. aureus. Little is known about the onset mechanism of deep tissue infection in AD. However, it is likely that patients with AD habitually scratch the skin due to severe itching, resulting in damage to the skin barrier which causes deep invasion of S. aureus.
It has been reported that patients with AD have decreased skin microbial diversity and dysbiosis compared to healthy subjects. Patients with AD have increased serine protease activity.3) Hyperactive serine protease responses appear to be responsible for increased desquamation of the skin, altered cathelicidin, and inflammation.3,14)
S. aureus serine proteases were directly linked to AD severity, and therefore, this dysbiosis was recovered after treatment.14,15) Analysis of skin microbiome in our 2 patients also demonstrated low diversity and high S. aureus loading, suggesting that the microbial landscape of skin may be related to skin immunity and AD.
Treatment of AD with appropriate corticosteroid ointments or emollients reduces the number of colonizing bacteria and the concurrent increase in microbial diversity.15,16) In our patients, bacterial infections were relatively limited to the breast tissue and no other skin or deep tissue infections were observed. Although we did not demonstrate a deficiency of antimicrobial peptide or a systemic immunodeficiency in our patients, we thought that the skin-localized immunodeficiency or dysbiosis of skin microbiome might contribute to this invasive infection.
AD associated with invasive S. aureus infection is probably underreported. A high rate of cutaneous colonization by S. aureus in AD lesions represents an important source of invasive bacterial infection. Our patients demonstrate the potential severity of bacterial infections in AD. Although breast abscess is rare in AD, it should be recognized as a possible complication in this common skin disorder.
Skin infection by S. aureus commonly occurs in patients with AD. Deep tissue infection is rare but a possible complication in patients with AD, and therefore clinicians should be aware of this complication.
Notes
Conflicts of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Tauber M, Balica S, Hsu CY, Jean-Decoster C, Lauze C, Redoules D, et al. Staphylococcus aureus density on lesional and nonlesinal skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol 2016;137:1272–1274. 1274.e1–1274.e3.


2. Benenson S, Zimhony O, Dahan D, Solomon M, Raveh D, Schlesinger Y, et al. Atopic dermatitis-a risk factor for invasive Staphylococcus aureus infections: two cases and review. Am J Med 2005;118:1048–1051.


3. Williams MR, Gallo RL. The role of skin microbiome in atopic dermatitis. Curr Allergy Asthma Rep 2015;15:65


5. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002;347:1151–1160.


6. Kitamura S, Nakayama Y, Shirai Y, Hashiguchi H, Kim R. Septic arthritis of the hip associated with atopic dermatitis. J Nippon Med Sch 2000;67:464–467.


7. Hoeger PH, Ganschow R, Finger G. Staphylococcal septicemia in children with atopic dermatitis. Pediatr Dermatol 2000;17:111–114.


8. Mohiyiddeen G, Brett I, Jude E. Infective endocarditis caused by Staphylococcus aureus in a patient with atopic dermatitis: a case report. J Med Case Rep 2008;4:143
9. Horimoto K, Kubo T, Matsusaka H, Baba H, Umesue M. Right-sided infective endocarditis with a ruptured sinus of Valsalva and multiple septic pulmonary emboli in a patient with atopic dermatitis. Intern Med 2015;54:797–800.


10. Gollapalli V, Liao J, Dudakovic A, Sugg SL, Scott-Conner CE, Weigel RJ. Risk factors for development and recurrence of primary breast abscesses. J Am Coll Surg 2010;211:41–48.


11. Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 2009;123:e808–e814.


12. Bunikowski R, Mielke MA, Skarabis H, Worm M, Anagnostopoulos I, et al. Evidence for a disease-promoting effect of Staphylococcus aureus-derived exotoxins in atopic dermatitis. J Allergy Clin Immunol 2000;105:814–819.


13. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev 2011;242:233–246.



14. Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 2012;22:850–859.



Fig. 1
Tense and swollen left breast (A) and a large abscess and adjacent inflammatory changes in the left subareolar area (B) were identified.
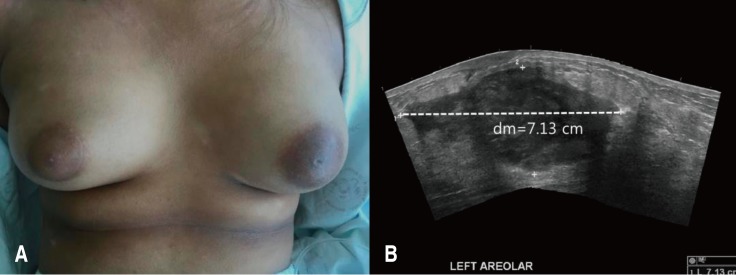
Fig. 2
(A) Matrix-assisted laser desorption/ionization time-of-flight (Maldi-ToF) analysis isolated identical Staphylococcus aureus strains from the breast abscess material as well as the lesional and the normal-appearing skin. (B) Skin microbiome analysis by next generation sequencing shows different diversities at the phylum level and abundant Staphylococcus species in the lesional skins in case 1. PCA, principal component analysis.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


