Introduction
Management of patent ductus arteriosus (PDA) including the most appropriate drug, the optimal timing and duration and the proper dosing regimens for pharmacological approach is still a matter of debate. Traditionally, 3 doses of ibuprofen or indomethacin have been given at regular intervals. However, ductal closure is observed by the time the second or third dose is given
1,2), hence, some investigators have used echocardiography-guided medical treatment to limit unnecessary exposure to cyclooxygenase inhibitors in preterm infants with PDA
3,4,5).
Plasma B-type natriuretic peptide (BNP) measurement have been proposed as a tool for the prediction and diagnosis of symptomatic PDA (sPDA) in preterm infants, especially using commercially available bedside testing kit
6,7,8,9). A recent systematic review evaluating the diagnostic accuracy of BNP for hemodynamically significant PDA (hsPDA) suggests that plasma BNP measurement seems to be an accurate test for identifying hsPDA in preterm infants
10).
We investigated the usefulness of serial BNP measurement as a guide for individual identification of early constrictive responses to ibuprofen. We hypothesized that BNP-guided pharmacological treatment can reduce the number of ibuprofen doses without affecting its efficacy and safety in very preterm infants with sPDA.
Materials and methods
This observational study was conducted in the neonatal intensive care unit (NICU) at Korea University Ansan Hospital from April 2008 to March 2014. Very preterm infants of <32 weeks gestation, who required respiratory support, received initial screening echocardiography within 24 hours of birth and were monitored for clinical symptoms of PDA.
Details of echocardiographic studies and diagnosis and mangement of sPDA in this study have been reported previously
6,8). The clinical signs of hsPDA was based on the following features: (1) the presence of a systolic or continuous murmur, (2) a bounding pulse or a hyperactive precordial pulse, (3) difficulty in maintaining the blood pressure, i.e., hypotension (the lower limit of normal mean arterial pressure was regarded to corrected gestational age) without response to loading fluid and infusion of dopamine, (4) a worsening ventilator status, and (5) chest radiographic evidence, i.e., pulmonary congestion or cardiomegaly (a cardiothoracic ratio >60%) with increased pulmonary flow.
sPDA was defined as the presence of 2 of these 5 signs with the confirmation of a large left to right ductal flow by color-flow Doppler echocardiography. The policy of management to PDA in our NICU was that cyclooxygenase inhibitor was administered intravenously after confirming the diagnosis of sPDA. If the sPDA failed to close with at least 2 courses of cyclooxygenase inhibitors or if pharmacological treatment was contraindicated, surgical ligation was performed immediately.
During Era 1 (before March 2010), the standard course of pharmacological treatment was initiated with indomethacin (or ibuprofen) and routinely followed by 2 additional doses of indomethacin (or ibuprofen) at intervals of 24 hours. During Era 2 (after April 2010), individualized pharmacological treatment was used, starting with the first dose of ibuprofen and witholding of additional ibuprofen doses if the BNP concentration was <600 pg/mL and clinical signs of hsPDA were improved before the next scheduled dose of ibuprofen.
Infants who received cyclooxygenase inhibitors prophylactically, who had Ōēźgrade 3 intraventricular hemorrhage (IVH) prior to pharmacological treatment and who had congenital heart disease or other congenital disease were excluded.
Echocardiography was conducted at 72 hours after the initiation of pharmacological treatement to confirm early ductal closure after the first course of treatment. And repeated echocardiographies were conducted for evaluations of ductal closure at discharge and final ductal clousre at 3 months after discharge. The response to pharmacological treatment is defined as an improvement of clinical symptoms of PDA and a decreased or no ductal shunt by echocardiography after the first or second course of treatment.
The BNP levels were assessed using a commercial kit (Triage BNP test; Alere Triage Meterpro, San Diego, CA, USA) by a fluorescence immunoassay at the bed side
9) and were measured before the first dose of pharmacological treatment and before the next scheduled dose of ibuprofen during Era 2.
BPD was defined according to the National Institutes of Health consensus definition
11). IVH was classified according to Papile et al.
12). NEC was classified according to Bell's classification, modified by Kliegman and Walsh
13).
Informed consents were obtained from all parents of infants included during Era 2. The study was approved by the local research ethics committee of Korea University Ansan Hospital.
The chi-square test or Fisher exact test was used to compare categorical variables and the Student t test or the Mann-Whitney rank sum U test to compare continuous variables. A P value below 0.05 was considered statistically significant.
Results
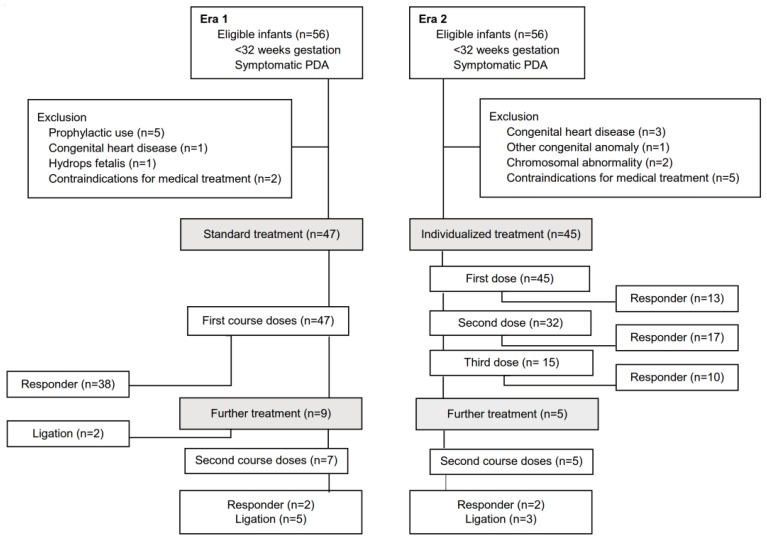
Forty-seven infants in Era 1 (standard group) and 45 infants in Era 2 (BNP-guided group) were eligible for the final analysis (
Fig. 1). Perinatal demographic variables were similar in the 2 groups (
Table 1).
The BNP-guided group received significantly fewer doses of ibuprofen than the standard group during the first course of treatment and the entire study period (
Table 2). Although the echocardiographic ductal closure rate at discharge was lower in the BNP-guided group, as compared to the standard group, there was no significant difference in the incidence of reappearance of sPDA after the first and/or second course of treatment. The need for further doses of cyclooxygenase inhibitors and the need for surgical ligation were not significantly different between the 2 groups. No significant differences were seen in clinical outcomes and/or complications related sPDA and/or pharmacological treatment, such as IVH, necrotizing enterocolitis, oliguria, bronchopulmonary dysplasia, and mortality. However, there was a significantly lower incidence of increased creatinine levels in the BNP-guided group.
The comparative efficacy and safety of the early constrictive response to cyclooxygenase inhibitors in the BNP-guided group, as compared with the standard group was determined by subanalysis for responders to the first course of treatment in both groups (
Table 3). Of 38 infants who responded to the first course of treatment in the standard group, 3 infants with incomplete dosing (2 doses) due to contraindications of the medication were excluded.
Discussion
Previous clinical trials showed that PDA treatment decisions based on serial echocardiographic assessment of ductal diameter
3) or flow pattern
4) was associated with reduction in total drug doses without compromising efficacy and safety. Recently, a randomized controlled clinical trial
5) for echocardiography-guided medical treatment for PDA in preterm infants showed that echocardiography-guided treatment is feasible and reduces unnecessary doses of ibuprofen without increasing the reopening rate. However, coherent echocardiographic evaluation by skilled clinicians is difficult to perform routinely, and the hemodynamic effects of PDA may be difficult to determine even with typical echocardiographic features.
Plasma BNP, emerging as a sensitive and specific biomarker of sPDA, rapidly decreases in infants as a response to cyclooxygenase inhibitors for ductal closure
9,10,14). Attridge et al.
15) reported that BNP levels can be used as a guide for the treatment of PDA and BNP-guided treatment reduces the number of indomethacin doses during the first course of treatment without any changes in the rate of PDA closure or complications. Furthermore, our study showed that, in addition to reducing the ibuprofen doses during the first course of treatment, BNP-guided treatment can reduce the total number of ibuprofen doses during the study periods without affecting the efficacy and safety for sPDA in very preterm infants.
In our subanalysis for responders to first course of treatment (
Table 3) to identify the efficacy and safety of the early constrictive response to ibuprofen in the BNP-guided group, clinical symptoms of PDA were improved earlier and BNP concentrations were rapidly decreased after the first or second dose of ibuprofen in the incomplete dosing group, as compared to the complete dosing groups. Echocardiographic early ductal closure rate (63.3%, 19 of 30) was higher in the incomplete dosing group, as compared to the complete dosing group. Three infants in the incomplete dosing group had recurrence of sPDA and received the further dose of ibuprofen after the first course of ibuprofen. Finally 2 infants required surgical ligation because the clinical symptoms were aggravated despite additional 2 and 4 doses of ibuprofen, respectively.
The incidences of early ductal closure and ductal closure at discharge were significantly lower in the incomplete dosing group and the complete dosing group in the BNP-guided group, as compared with the complete dosing group of the standard group. Nevertheless, there were no significant differences in the incidence of reappearance of sPDA, the need for a further dose of cyclooxygenase inhibitors and surgical ligation and the final ductal closure rate between the responders to first course of treatment in the BNP-guided group as compared with that of the standard group.
This is an observational study demonstrating that BNP-guided pharmacological treatment may be used clinically to avoid unnecessary doses of cyclooxygenase inhibitors. However, in addition to a cutoff level <600 pg/mL of BNP, the clinical improvement of symptoms of PDA was concurrently used for individualized pharmacological treatment of sPDA in our study. We assumed that even if the clinical symptoms of PDA are improved, the high BNP level represent that the infant still have an hsPDA and is required additional dose of medication.
Moreover, we estimated that a BNP level lower than 600 pg/mL, half value of the best cutoff BNP levels (1,100 pg/mL) for the diagnosis of sPDA in our previous study
6), was used as a guide for individualized treatment. To further investigate the diagnostic utility of a rapid BNP assay for the identification of the early constrictive response to ibuprofen, the best cutoff value of BNP should be determined by the follow-up study in the BNP-guided group.
In conclusion, serial BNP measurement during the management of sPDA can be used as a guide for early constrictive responses to cyclooxygenase inhibitors. Individualized BNP-guided pharmacological treatment may be used clinically to avoid the unnecessary use of cyclooxygenase inhibitors in infants without increasing the incidence of ductal closure failure and the short-term morbidity related sPDA. A large multicenter randomized controlled trial, especially, with long-term follow-up on developmental outcomes is needed to determine if this approach should be a standard management practice for sPDA.
Acknowledgments
We thank the physicians and nursing staff working in the NICU of Korea University Ansan Hospital for their enthusiastic support and cooperation.
Notes
Conflict of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Dumas de la Roque E, Fayon M, Babre F, Demarquez JL, Pedespan L. Minimal effective dose of indomethacin for the treatment of patent ductus arteriosus in preterm infants. Biol Neonate 2002;81:91ŌĆō94.


2. Su BH, Lin HC, Chiu HY, Hsieh HY, Chen HH, Tsai YC. Comparison of ibuprofen and indometacin for early-targeted treatment of patent ductus arteriosus in extremely premature infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2008;93:F94ŌĆōF99.


3. Carmo KB, Evans N, Paradisis M. Duration of indomethacin treatment of the preterm patent ductus arteriosus as directed by echocardiography. J Pediatr 2009;155:819ŌĆō822.e1.


5. Bravo MC, Caba├▒as F, Riera J, P├®rez-Fern├Īndez E, Quero J, P├®rez-Rodr├Łguez J, et al. Randomised controlled clinical trial of standard versus echocardiographically guided ibuprofen treatment for patent ductus arteriosus in preterm infants: a pilot study. J Matern Fetal Neonatal Med 2014;27:904ŌĆō909.


6. Choi BM, Lee KH, Eun BL, Yoo KH, Hong YS, Son CS, et al. Utility of rapid B-type natriuretic peptide assay for diagnosis of symptomatic patent ductus arteriosus in preterm infants. Pediatrics 2005;115:e255ŌĆōe261.


8. Lee JH, Shin JH, Park KH, Rhie YJ, Park MS, Choi BM. Can early B-type natriuretic peptide assays predict symptomatic patent ductus arteriosus in extremely low birth weight infants? Neonatology 2013;103:118ŌĆō122.


10. Kulkarni M, Gokulakrishnan G, Price J, Fernandes CJ, Leeflang M, Pammi M. Diagnosing significant PDA using natriuretic peptides in preterm neonates: a systematic review. Pediatrics 2015;135:e510ŌĆōe525.


11. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723ŌĆō1729.


12. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529ŌĆō534.


13. Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 1987;17:213ŌĆō288.

14. Lee EH, Choi BM. Clinical applications of plasma B-type natriuretic peptide assays in preterm infants with patent ductus arteriosus. Neonatal Med 2013;20:323ŌĆō334.

15. Attridge JT, Kaufman DA, Lim DS. B-type natriuretic peptide concentrations to guide treatment of patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed 2009;94:F178ŌĆōF182.


Fig.┬Ā1
Flow diagram of study subject enrollment and responses of patent ductus arteriosus (PDA) to the first and second course of medical treatment during Era 1 (before March 2010; standard group) and Era 2 (after April 2010; BNP-guided group).
Table┬Ā1
Perinatal demographic variables of standard treatment and individualized BNP-guided treatment groups
![kjped-60-175-i001.jpg]()
|
Variable |
Standard treatment (n=47) |
Individualized BNP-guided treatment (n=45) |
P value |
|
Gestational age (wk) |
27.9┬▒2.1 |
28.0┬▒2.3 |
0.940 |
|
Birth weight (g) |
1,019┬▒299 |
1,105┬▒369 |
0.225 |
|
Male sex |
23 (48.9) |
17 (37.8) |
0.280 |
|
Cesarean section |
31 (66.0) |
34 (75.6) |
0.312 |
|
Apgar score |
|
|
|
|
ŌĆā1 Minute |
4 (3ŌĆō6) |
5 (3ŌĆō6) |
0.912 |
|
ŌĆā5 Minutes |
7 (5.5ŌĆō8) |
7 (5.5ŌĆō8) |
0.418 |
|
PROM Ōēź 18 hours |
6 (12.8) |
12 (26.7) |
0.093 |
|
Preeclampsia |
14 (29.8) |
7 (15.6) |
0.104 |
|
Completed antenatal steroid |
18 (38.3) |
22 (48.9) |
0.306 |
|
Surfactant treatment |
43 (91.5) |
45 (100) |
0.117 |
|
Noninvasive ventilation at the 1st dose of treatment |
5 (10.6) |
5 (11.1) |
0.942 |
|
Invasive ventilation at the 1st dose of treatment |
42 (89.4) |
40 (88.9) |
0.942 |
Table┬Ā2
Neonatal outcomes of standard treatment group and individualized BNP-guided treatment group
![kjped-60-175-i002.jpg]()
|
Variable |
Standard treatment (n=47) |
Individualized BNP-guided treatment (n=45) |
P value |
|
Cyclooxygenase inhibitors treatment |
|
|
|
|
ŌĆāIndomethacin:Ibuprofen |
32:15 |
0:45 |
<0.001 |
|
ŌĆāAge at the 1st dose of treatment (hr) |
48.1┬▒17.9 |
48.4┬▒16.5 |
0.912 |
|
ŌĆāDoses during the 1st course |
2.9┬▒0.4 |
2.0┬▒0.8 |
<0.001 |
|
ŌĆāTotal doses during the study period |
3.5┬▒1.2 |
2.9┬▒2.2 |
<0.001 |
|
PDA outcomes |
|
|
|
|
ŌĆāResponse to 1st course |
38 (80.9) |
40 (88.9) |
0.283 |
|
ŌĆāResponse to 2nd course |
2 (4.3) |
2 (4.4) |
0.999 |
|
ŌĆāReappearance of symptomatic PDA |
3 (6.4) |
5 (11.1) |
0.481 |
|
ŌĆāNeed for further dose of treatment |
2 (4.3) |
7 (15.6) |
0.087 |
|
ŌĆāNeed for surgical ligation |
10 (21.3) |
7 (15.6) |
0.480 |
|
Echocardiographic examination |
|
|
|
|
ŌĆāEarly ductal closure |
28 (59.6) |
20 (44.4) |
0.146 |
|
ŌĆāDuctal closure at discharge |
46 (97.9) |
38 (84.4) |
0.029 |
|
ŌĆāFinal ductal closure after discharge |
46 (97.9) |
45 (100) |
0.999 |
|
Clinical outcomes |
|
|
|
|
ŌĆāDuration of invasive ventilation (day) |
12 (5ŌĆō39) |
9 (3ŌĆō31) |
0.444 |
|
ŌĆāDuration of hospital (day) |
76 (60ŌĆō99) |
76 (55ŌĆō113) |
0.553 |
|
ŌĆāIVH, Ōēźgrade 3 |
8 (17.0) |
2 (4.4) |
0.091 |
|
ŌĆāNEC, Ōēźgrade II |
2 (4.3) |
2 (4.4) |
0.999 |
|
ŌĆāOliguria, <1 mL/kg/hr |
2 (4.3) |
1 (2.2) |
0.999 |
|
ŌĆāCreatinine, Ōēź1.2 mg/dL |
19 (48.7) |
8 (19.0) |
0.005 |
|
ŌĆāBPD, Ōēźmoderate |
24 (51.1) |
16 (35.6) |
0.134 |
|
ŌĆāMortality |
2 (4.3) |
2 (4.4) |
0.999 |
Table┬Ā3
Outcomes of the responders to the first course of pharmacological treatment in the standard and individualized BNP-guided groups
![kjped-60-175-i003.jpg]()
|
Variable |
Standard treatment |
Individualized BNP-guided treatment |
|
1st course of cyclooxygenase inhibitors |
1st course of ibuprofen |
2nd course of ibuprofen |
|
Complete dosing |
Incomplete dosing |
Complete dosing |
|
3 Doses (n=35) |
1 Dose (n=13) |
2 Doses (n=17) |
3 Doses (n=10) |
>3 Doses (n=5) |
|
Cyclooxygenase inhibitors treatment |
|
|
|
|
|
|
ŌĆāAge at the 1st dose of treatment (hr) |
49.1┬▒18.1 |
52.4┬▒11.0 |
47.3┬▒10.9 |
53.9┬▒26.7 |
31.2┬▒6.3 |
|
ŌĆāDoses during the 1st course |
3 |
1 |
2 |
3 |
3 |
|
ŌĆāTotal doses during the study period |
3.1┬▒1.9 |
1.4┬▒1.4 |
2.4┬▒1.1 |
3.3┬▒0.9 |
7.6┬▒2.1 |
|
PDA outcomes |
|
|
|
|
|
|
ŌĆāReappearance of symptomatic PDA |
2 (5.7) |
1 (7.7) |
2 (11.8) |
2 (20) |
- |
|
ŌĆāNeed for further dose of cyclooxygenase inhibitors |
2 (5.2) |
1 (7.7) |
2 (11.8) |
1 (10) |
3 (60) |
|
ŌĆāNeed for surgical ligation |
2 (5.2) |
0 |
2 (11.8) |
1 (10) |
4 (80) |
|
Echocardiographic examination |
|
|
|
|
|
|
ŌĆāEarly ductal closure |
27 (77.1) |
10 (76.9) |
9 (52.9) |
1 (10) |
0 |
|
ŌĆāDuctal closure at discharge |
35 (100) |
11 (84.6) |
15 (88.2) |
7 (70) |
4 (80) |
|
ŌĆāFinal ductal closure after discharge |
35 (100) |
13 (100) |
17 (100) |
10 (100) |
5 (100) |
|
BNP concentrations (pg/mL) |
|
|
|
|
|
|
ŌĆāBefore the 1st dose |
- |
2,085┬▒1,162 |
2,341┬▒1,308 |
2,093┬▒1,185 |
3,070┬▒1,152 |
|
ŌĆāAfter 24 hours after 1st dose |
- |
201┬▒193 |
654┬▒937 |
1,182┬▒1,129 |
3,200┬▒1,769 |
|
ŌĆāAfter 24 hours after 2nd dose |
- |
- |
145┬▒146 |
537┬▒336 |
2,938┬▒2,172 |
|
ŌĆāAfter 24 hours after 3rd dose |
- |
- |
- |
274┬▒198 |
2,919┬▒1,976 |




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


