Article Contents
| Clin Exp Pediatr > Volume 62(3); 2019 |
|
Abstract
Purpose
Methods
Results
Fig. 1.
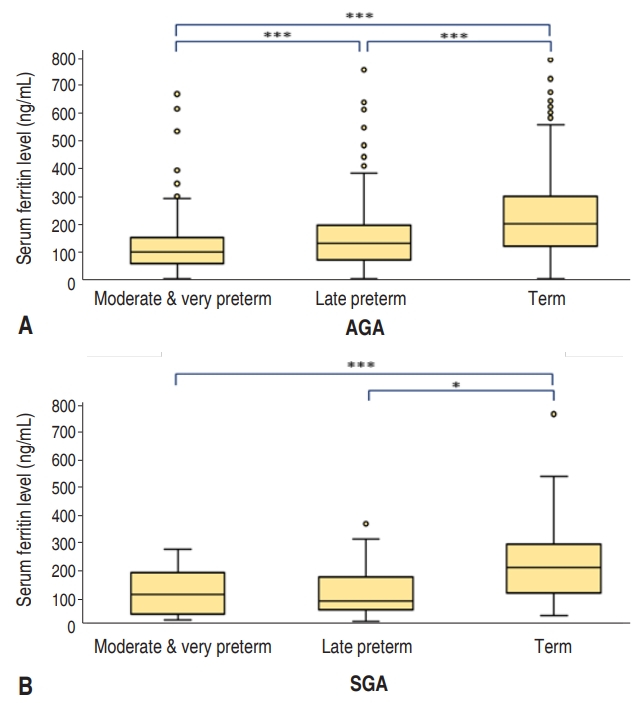
Table 1.
Values are presented as number (%) or mean±standard deviation unless otherwise indicated.
SGA, small for gestational age; AGA, appropriate for gestational age; GA, gestational age; c-sec, cesarean section; IQR, interquartile range; ROM, rupture of membranes; GDM, gestational diabetes; DM, diabetes mellitus; PIH, pregnancy induced hypertension.
Table 2.
Table 3.
Values are presented as median (interquartile range).
SGA, small for gestational age; AGA, appropriate for gestational age; c-sec, cesarean section; ROM, rupture of membranes; GDM/DM, gestational diabetes/diabetes mellitus; PIH, pregnancy induced hypertension; HTN, hypertension; HC, head circumferences; Yes, affected by each factor; No, not affected by each factor.
Table 4.
Values are presented as median (interquartile range) for unadjusted.
SGA, small for gestational age; AGA, appropriate for gestational age; c-sec, cesarean section; GDM/DM, gestational diabetes/diabetes mellitus; PIH, pregnancy induced hypertension; HTN, hypertension; HC, head circumferences; Yes, affected by each factor; No, not affected by each factor.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


