Article Contents
| Clin Exp Pediatr > Volume 54(4); 2011 |
Abstract
Purpose
Acute internal hemorrhage is an occasionally life-threatening complication in pediatric cancer patients. Many therapeutic approaches have been used to control bleeding with various degrees of success. In this study, we evaluated the efficacy of selective internal iliac artery embolization for controlling acute intractable bleeding in children with malignancies.
Methods
We retrospectively evaluated the cases of 6 children with various malignancies (acute lymphoblastic leukemia, acute myelogenous leukemia, chronic myelogenous leukemia, T-cell prolymphocytic leukemia, Langerhans cell histiocytosis, and rhabdomyosarcoma), who had undergone selective arterial embolization (SAE) of the internal iliac artery at the Chonnam National University Hwasun Hospital between January 2004 and December 2009. SAE was performed by an interventional radiologist using Gelfoam® and/or Tornado® coils.
Results
The patients were 5 boys and 1 girl with median age of 6.9 years (range, 0.7-14.8 years) at the time of SAE. SAE was performed once in 4 patients and twice in 2, and the procedure was unilateral in 2 and bilateral in 4. The causes of hemorrhage were as follows: hemorrhagic cystitis (HC) in 3 patients, procedure-related internal iliac artery injuries in 2 patients, and tumor rupture in 1 patient. Initial attempt at conservative management was unsuccessful. Of the 6 patients, 5 (83.3%) showed improvement after SAE without complications.
Acute hemorrhage can be a life-threatening complication in cancer patients and is a significant cause of morbidity and mortality1-3). It occurs in approximately 6-10% of cancer patients3, 4). Medical treatment for acute hemorrhage includes compression, drug therapy, blood transfusion, hydration and other supportive care. Other therapeutic strategies include radiotherapy, surgical therapy, such as vessel ligation or the resection of hemorrhagic tissue3). However, the inherent morbidity of the surgical procedures in debilitated patients, and the efficacy of medical treatment may make this approach problematic3, 5). Therefore, selective arterial embolization (SAE) is increasingly preferred as a therapeutic procedure for controlling hemorrhage, normally in adults. Furthermore, SAE is applied increasingly in conditions, such as hemorrhage, arterio-venous malformation, arteriovenous fistula, aneurysm and tumors6-9). However, SAE is performed less frequently in pediatric patients. This may be secondary to the stress of the procedure itself, lack of doctor`s experience and possible long-term side effects8). Therefore, the aim of this retrospective study was to determine the efficacy and safety of SAE in the treatment of intractable hemorrhage in pediatric patients with malignancies.
From January 2004 to December 2009, the medical records of 6 pediatric patients with a malignancy who underwent SAE of the internal iliac artery at Chonnam National University Hwasun Hospital were analyzed retrospectively. All patients presented with intractable hemorrhage, which had not responded to earlier conservative measures. Data on the following items were documented: general characteristics, underlying disease, treatment of underlying disease, number of SAE performed, response to SAE, side effects related to the procedure and follow-up duration. The complications related to the SAE procedure, including nausea, vomiting, fever, development of hematoma, infection, vascular perforation, ischemia, and pain were also examined. SAE was performed by an interventional radiologist under fluoroscopic guidance after patients had been appropriately sedated. The Gelfoam® (Pfizer Inc., New York, NY, USA) and/or Tornado® coils (Cook Inc., Bloomington, IN, USA) were used as embolic agents.
In cases of hemorrhagic cystitis (HC), the onset time and duration of HC before SAE were reviewed. HC was graded according to the criteria previously reported9): grade 1, continuation of microscopic hematuria for more than 2 days; grade 2, gross hematuria; grade 3, gross hematuria with a clot; grade 4, gross hematuria with renal impairment due to urinary tract obstruction with a clot.
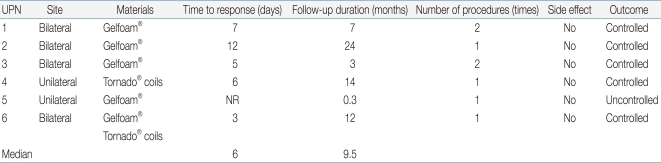
Six patients with various malignancies were enrolled in this study: acute lymphocytic leukemia, acute myelogenous leukemia, chronic myelogenous leukemia, T-cell prolymphocytic leukemia, Langerhans cell histiocytosis and rhabdomyosarcoma. The median age was 6.9 years (range, 0.7-14.8 years). Five were males (83.3%). The median follow-up period was 9.5 months (range, 0.3-24 months). The patient with rhabdomyosarcoma was treated by chemotherapy consisting of vincristine, dactinomycin and cyclophosphamide. The other 5 patients (83.3%) received allogeneic hematopoietic stem cell transplantation (HSCT). The stem cell sources for transplantation were umbilical cord blood, unrelated bone marrow and unrelated peripheral blood for 1 patient each and haploidentical bone marrow for 2 patients (Table 1).
In 3 of the 6 patients (50%), the cause of the hemorrhage was HC, which developed after allogeneic HSCT. Two patients (33.3%) had an iatrogenic iliac artery injury and the last patient had a tumor rupture (Table 1). For the children with HC, the median day of onset was 15 days after allogeneic HSCT (range, 13-47 days), and the median duration of HC was 15 days (range, 9-33 days) before SAE. Two patients had grade 3 HC and 1 had grade 4. For transplant conditioning, intravenous busulfan and cyclophosphamide were used in 2 patients with HC, and cyclophosphamide and total body irradiation were used in 1. Two patients were positive for both adenovirus type 2 and the BK virus in blood and urine, which are associated with HC.
Before SAE was performed, numerous therapeutic approaches had been attempted. In cases of HC, all the measures, such as forced hydration over 3000 mL/m2 per day, platelet or red cell and fresh frozen plasma transfusions, Foley catheter insertion, instillation of sodium hyaluronate within the bladder, diuretics and antiviral agents were not able to control the bleeding (Table 2). Three patients with refractory hemorrhage due to internal iliac artery injury or tumor rupture did not respond to medical treatments including hydration, administration of blood products, inotropic agents and vitamin K administration, resulting in hypotension and markedly decreased hemoglobin (Hb) concentration (mean value, 5.1 g/dL). Surgery was generally not attempted because of high risk conditions, such as, low platelet counts less than 20,000/mm3, prolonged PT/PTT and decreased neutrophil counts. In addition, active bleeding sites were able to be localized by angiography. Thus, SAE was attempted instead of surgery.
Of the 6 patients, 4 (66.7%) underwent bilateral SAE, and 2 (33.3%) had unilateral SAE. Gelfoam® and/or Tornado® coils were used for embolization (Gelfoam® alone in 4 patients; Tornado® coils alone in 1; both Gelfoam® and Tornado® coils in 1). Five (83.3%) patients responded to SAE initially but repeat SAE was needed because the hemorrhage reappeared in 2 patients. There were no more recurrences after the repeated procedures. After SAE, it took a median interval of 6 days (range, 3-12 days) to control the bleeding. One patient who did not respond to SAE in addition to supportive measures died of hypotension caused by the uncontrolled bleeding 3 days after the procedure. During follow-up period, two more patients died from causes not related to bleeding: chronic graft-versus-host disease 14 months after HSCT; and sepsis 3 months after transplantation.
In cancer patients, acute hemorrhage is a well-known complication that is associated with high morbidity and mortality3, 5). Various therapeutic approaches have been attempted including intensive supportive care with compression, hydration, blood transfusion, drug therapy and surgical care3, 5, 9). However, there is no specific and effective treatment. Some patients do not respond to medical care5, 11). Surgical treatment is the ultimate therapeutic approach for controlling refractory hemorrhage but is associated with high morbidity and mortality because of the poor general condition of the cancer patients or underlying disease as well as the complications arising from treatment of the underlying disease2, 12). SAE is a minimally invasive procedure with a lower rate of morbidity, having obvious advantages over surgery. Therefore, it is an accepted approach and is used more often to treat intractable hemorrhage5, 12, 13).
The SAE of the internal iliac artery was first reported in 1973 as a mean to control the bleeding associated with pelvic fractures16). In 1974, Halad and Myging17) were the first to describe the use of SAE of the internal iliac artery to control HC secondary to radiation therapy for bladder cancer. Since then, the indications for SAE of the internal iliac artery to control intractable hemorrhage have widened18), including the management of hemorrhage resulting from trauma, iatrogenic or obstetrical causes, and pelvic or urological neoplasm2, 5, 9, 14, 15, 19). In this study, all 6 children had an acute pelvic cavity hemorrhage resulting from HC, iatrogenic vascular injuries or rupture of a retroperitoneal tumor, which could not be controlled by medical treatment. SAE of the internal iliac artery was attempted as surgical therapy was impossible due to the poor general condition of the patients and their low platelet counts. The bleeding was brought under control in 5 of the 6 patients (83.3%) after SAE. The remaining one patient died of uncontrollable bleeding despite the SAE procedure because of poor general condition associated with underlying hypotensive shock. These results are similar to those of previous studies showing the efficacy of SAE2, 5, 9, 11, 12, 14, 15).
According to Han et al.5), 10 patients with grade 3-4 HC following allogeneic HSCT underwent SAE of the internal iliac artery. The unsuccessful medical treatments performed before SAE included the following: hyperhydration, blood transfusion, intravesical instillation of granulocyte, monocyte-colony stimulating factor, bladder irrigation, and antiviral agents. Eight patients responded to SAE within 15 days. Surgical treatment, such as cauterization with cystoscopy or cystectomy, was not necessary5). There were several reports about successful SAE; 2 adults with HC who received an allogeneic HSCT2) and 6 patients (mean age 80 years) with bladder or prostate carcinoma, who all patients were embolized successfully within 8 days and without complications12). SAE was also performed successfully in instances of severe postpartum hemorrhage, pelvic fracture or trauma15, 20).
During the follow-up period, 2 children underwent a second SAE due to a relapse of the hemorrhage but there were no recurrences after the second procedure. This finding is comparable to that of other studies. In the recent study, 6 adult patients were reported who underwent selective embolization of the bladder arteries for treatment of incoercible hematuria. All patients with hematuria responded within 48 hours after the procedure, but two patients had a recurrence of the bleeding that required a second procedure21). In 2007 report, among 7 patients with intractable bladder hemorrhage who underwent internal iliac artery embolization, but bleeding was controlled permanently in 4 (57%) patients and a second embolization was required in 1 patient22). In the other study of 44 patients with an advanced pelvic tumor (mean age 79 years; range 51-95), the initial embolization produced complete control of the bleeding in 36 of the 44 patients (82%). However, subsequent arterial embolization was required in 5 patients (11%) and was successful23). In addition, this procedure can also be used to diagnose and treat aneurysms, arterio-venous malformation or tumors6, 24, 25).
However, SAE is performed less frequently in children, particularly in pediatric cancer patients6-8, 24). A search of the literature on SAE in the pediatric population revealed only a few reports. Legge et al.8) performed SAE in 5 pediatric patients suffering from hypersplenism, hemobilia, sigmoid hemangioma and aneurysmal bone cysts in the iliac bone. Embolization provided definitive treatment in all cases without complications. Bilateral internal pudendal artery embolization was performed for intractable priapism in a 14-year-old boy with chronic myelogenous leukemia7). A month-old baby with a mycotic internal iliac aneurysm underwent successful coil embolization6). Erkan24) also carried out SAE for the treatment of pelvic aneurysmal bone cysts in two children. Along with the reported cases, our data on 6 patients support the effectiveness of SAE for the treatment of refractory hemorrhage in children with a malignancy.
A range of materials have been used for embolization including muscle, autologous clots, gelatin, coil, and balloon26). Gelfoam® and/or coil were used for embolization. Gelfoam®, a gelatin sponge, is a water-insoluble hemostatic material that is easy to handle. It is biodegradable or absorbable, and the vessel recanalizes within a few weeks. Consequently, it has fewer complications of vascular occlusion than using coil or surgical vessel ligation8, 26). A coil is effective either for fast flowing vessels or when needed to occlude a vessel permanently23).
The complications associated with SAE include nausea, vomiting, fever, infection, pain, hematoma, vascular perforation, chills, claudication, erectile dysfunction, urinary retention, and paresthesia5, 12, 17, 26-28). However, serious side effects are rare26, 29) and can be reduced by precise placement of the coil or Gelfoam® to preserve tissue perfusion12, 21, 30). In the current study, there were no side effects encountered during the follow-up period, but it will be necessary to carefully observe these patients for possible long-term complications, such as claudication, sexual dysfunction and paresthesia26-28).
In conclusion, bleeding was controlled in 5 out of 6 children (83.3%) after SAE of the internal iliac artery without side effects. The results suggest that SAE of the internal iliac artery is an effective and safe therapeutic approach in pediatric cancer patients with hemorrhage who do not respond to supportive care. SAE can also reduce the patients` exposure to the risks inherent with the surgical procedures. Further clinical trials involving a larger number of patients will be needed to evaluate the potential role, safety and possible long-term side effects of SAE in children with malignancies.
References
1. El-Zimaity M, Saliba R, Chan K, Shahjahan M, Carrasco A, Khorshid O. Hemorrhagic cystitis after allogeneic stem cell transplantation: donor type matters. Blood 2004;103:4674ŌĆō4680.


2. Gine E, Rovira M, Real I, Burrel M, Montana J, Carreras E, et al. Successful treatment of severe hemorrhage cystitis after hemopoietic cell transplantation by selective embolization of the vesical arteries. Bone Marrow Transplant 2003;31:923ŌĆō925.


3. Pereira J, Phan T. Management of bleeding in patients with advanced cancer. Oncologist 2004;9:561ŌĆō570.


4. Pereira J, Mancini I, Bruera E. Portenoy RK, Bruera E,The management of bleeding in patients with advanced cancer. editors. Topics in palliative care. 2000;Volume 4. New York: Oxford University Press, :163ŌĆō183.
5. Han Y, Wu D, Sun A, Xie A, Xu J, Zhou J, et al. Selective embolization of the internal iliac arteries for the treatment of severe hemorrhagic cystitis following hematopoietic SCT. Bone Marrow Transplant 2008;41:881ŌĆō886.


6. Khandanpour N, Chaudhuri A, Roebuck DJ, Armon MP. Neonatal mycotic internal iliac aneurysm due to methicillin-resistant Staphylococcus aureus (MRSA) septicaemia successfully treated by coil embolisation. Eur J Vasc Endovasc Surg 2007;33:687ŌĆō689.


7. Murayama K, Shibuya A, Ishii S, Sasaki N. Embolization of the bilateral internal pudendal arteries for intractable priapism in a child with chronic myelogenous leukemia. Rinsho Ketsueki 2001;42:1117ŌĆō1121.

9. Lang EK. Transcatheter embolization of pelvic vessels for control of intractable hemorrhage. Radiology 1981;140:331ŌĆō339.


10. Vela-Ojeda J, Tripp-Villanueva F, Sanchez-Cortes E, Ayala-Sanchez M, Garcia-Ruiz Esparza MA, Rosas-Cabral A, et al. Intravesical rhGM-CSF for the treatment of late onset hemorrhagic cystitis after bone marrow transplant. Bone Marrow Transplant 1999;24:1307ŌĆō1310.


11. Palandri F, Bonifazi F, Rossi C, Falcioni S, Arpinati M, Giannini MB, et al. Successful treatment of severe hemorrhagic cystitis with selective vesical artery embolization. Bone Marrow Transplant 2005;35:529ŌĆō530.


12. Nabi G, Sheikh N, Greene D, March R. Therapeutic transcatheter arterial embolization in the management of intractable haemorrhage from pelvic urological malignancies: preliminary experience and long-term follow-up. BJU Int 2003;92:245ŌĆō247.


13. Koc S, Hagglund K, Ireton R, Perez-Simon JA, Collins SJ, Appelbaum FR. Successful treatment of severe hemorrhagic cystitis with cystectomy following matched donor allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2000;26:899ŌĆō901.


14. Soyer P, Fargeaudou Y, Morel O, Boudiaf M, Dref OL, Rymer R. Severe postpartum haemorrhage from ruptured pseudoaneurysm: successful treatment with transcatheter arterial embolization. Eur Radiol 2008;18:1181ŌĆō1187.


15. T├Čtterman A, Dormagen JB, Madsen JE, Kl├Ėw NE, Skaga NO, R├Ėise O. A protocol for angiographic embolization in exsanguinating pelvic trauma: a report on 31 patients. Acta Orthop 2006;77:462ŌĆō468.


16. Ring EJ, Athanasoulis C, Waltman AC, Margolies MN, Baum S. Arteriographic management of hemorrhage following pelvic fracture. Radiology 1973;109:65ŌĆō70.


17. Hald T, Myging T. Control of life threatening vesical haemorrhage by unilateral hypogastric artery muscle embolization. J Urol 1974;112:60ŌĆō63.


18. Pisco JM, Martins JM, Correia MG. Internal iliac artery: embolization to control hemorrhage from pelvic neoplasms. Radiology 1989;172:337ŌĆō339.


19. Ratnam LA, Gibson M, Sandhu C, Chandraharan E, Belli AM. Transcatheter pelvic arterial emobolisation for control of obstetric and gynaecological haemorrhage. J Obstet Gynaecol 2008;28:573ŌĆō579.


20. Pieri S, Agresti P, Morucci M, De' Medici L, Galluzzo M, Oransky M, et al. Percutaneous management of hemorrhage in pelvic fractures. Radiol Med 2004;107:241ŌĆō251.

21. Palma Ceppi C, Reyes Osorio D, Palma Ceppi R, Palavecino P. Experience in superselective emolization of bladder arteries in the treatment of incoercible hematuria. Actas Urol Esp 2008;32:542ŌĆō545.


22. El-Assmy A, Mohsen T. Internal iliac artery embolization for the control of severe bladder hemorrhage secondary to carcinoma: long-term follow-up. ScientificWorldJournal 2007;7:1567ŌĆō1574.



23. Liguori G, Amodeo A, Mucelli FP, Patel H, Marco D, Belgrano E, et al. Intractable haematuria: long-term results after selective embolization of the internal iliac arteries. BJU Int 2010;106:500ŌĆō503.


24. Yildirim E, Ers├Čzl├╝ S, Kirbas I, Ozg├╝r AF, Akkaya T, Karadeli E. Treatment for pelvic aneurysmal bone cyst in two children: selective arterial embolization as an adjunct to curettage and bone grafting. Diagn Interv Radiol 2007;13:49ŌĆō52.

25. Bittles MA, Hoffer FA. Interventional radiology and the care of the pediatric oncology patient. Surg Oncol 2007;16:229ŌĆō233.


26. Travis T, Monsky WL, London J, Danielson M, Brock J, Wegelin J, et al. Evaluation of short-term and long-term complication after emergent internal iliac artery embolization in patients with pelvic trauma. J Vasc Interv Radiol 2008;19:840ŌĆō847.


27. Suzuki T, Shindo M, Kataoka Y. Clinical characteristics of pelvic fracture patients with gluteal necrosis resulting form transcatheter arterial embolization. Arch Orthop Trauma Surg 2005;125:448ŌĆō452.


28. Rayt HS, Bown MJ, Lambert KV, Fishwick NG, McCarthy MJ, Sayers RD. Buttock claudication and erectile dysfunction after internal iliac artery embolization in patients prior to endovascular aortic aneurysm repair. Cardiovasc Intervent Radiol 2008;31:728ŌĆō734.


Fig.┬Ā1
Image of the vesical arteries before and after embolization (Patient 1). A) Selective angiography of the right internal iliac artery before embolization shows prominent hypervascularization at the level of the left vesical arteries. B) Angiography findings of the internal iliac artery after embolization with Gelfoam® show improved hypervascularization of the left vesical arteries.
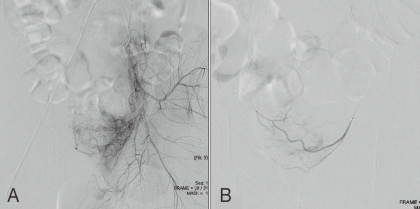
Fig.┬Ā2
Angiography showing the right internal iliac artery in patient 4. A) Selective angiography revealed active bleeding from the right internal iliac artery (arrow) before embolization. B) The bleeding was markedly reduced after selective internal iliac artery embolization with multiple microcoils.

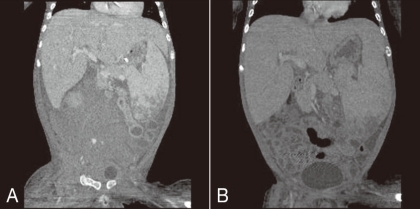
Fig.┬Ā3
A) Abdominal computerized tomography scan showing a large retroperitoneal hematoma and active bleeding in the iliopsoas area of the right pelvis due to an iatrogenic internal arterial injury (patient 4). B) Fourteen days after selective internal arterial embolization, right pelvic and lower abdominal retroperitoneal hematoma was markedly reabsorbed.
Table┬Ā1
General Patient Characteristics and the Cause of Internal Pelvic Cavity Hemorrhage

Abbreviations: UPN, unique patient number; M, male; F, female; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; T-PLL, T-cell prolymphocytic leukemia; ALL, acute lymphocytic leukemia; LCH, Langerhans cell histiocytosis; IRS, Inter-group rhabdomyosarcoma study; HSCT, hematopoietic stem cell transplantation; BM, bone marrow; UCB, umbilical cord blood; PB, peripheral blood; HC, hemorrhagic cystitis.
Table┬Ā2
Characteristics of Patients with Hemorrhagic Cystitis

Supportive care: hydration, transfusion, Foley catheter insertion, diuretics
Abbreviations: UPN, unique patient number; HC, hemorrhagic cystitis; SAE, selective arterial embolization; HSCT, hematopoietic stem cell transplant; TBI, total body irradiation; PCR, polymerase chain reaction; SH, sodium hyaluronate.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


